Telomerase promotes MSC immortalization and, in conjunction with additional events, produces cell transformation. These additional events usually implied an oncogene deregulation. One of the most important oncogenes involved in MSC transformation is c-myc. In our spontaneous model, senescent and post-senescence MSC, as well as TMC, overexpress c-myc. Consistent with our previous results, data from other groups have shown that c-myc seems to be essential to spontaneously transform MSC. In this regard, Funes et al. used retroviral vectors to introduce human telomerase, HVP-16 E6 and E7, H-Ras and SV40 small T antigen, individually or in combination, in human MSC. The combination of TERT, E6, E7 and H-Ras did not induce MSC transformation. Only MSC transduced with ST becomes transformed cells. ST inactivates phosphatase 2A, resulting in c-myc stabilization, suggesting that c-myc might be 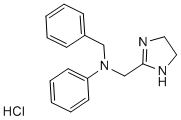 necessary to transform MSC. We explored DNA repair mechanisms to elucidate their role in MSC transformation. Post-senescent MSC showed downregulation of DNA-PKcs, ERCC3 and Rad51 proteins, each of which is associated to a distinct DNA repair pathway. Extremely restricted clonal selection takes place during cell crisis, and only cells with functional DNA repair mechanisms would continue to grow. TMC have a higher metabolic rate and divide more rapidly than pre- or post-senescence MSC, with a consequent increase in DNA damage. Proteins that participate in DNA repair are upregulated in TMC compared to MSC; this, together with telomere length maintenance, could permit cell survival, despite oxidative damage to DNA and be responsible for TMC karyotype stabilization. Recently it has been published the dependency on oxidative phosphorylation during MSC transformation. We have not detected statistically significant changes of these genes in our microarray experiments, although potential pathways leading to changes in postsenescence MSC and TMC revealed change in stress, toxic events and mitochondrial metabolism pathways. The definitive role of mitochondrial respiration on spontaneous MSC transformation remains to be investigated. A chromosome 5 alteration and a translocation are recurrent, stable features of in vitro cultured TMC. The telomerase gene map to human chromosome 5, suggests that it is activated by internal amplification of this chromosome in TMC. Chromosome 11 alterations are recurrent in tumors. Although we did not detect a target gene in the 3;11 translocation in our model, genes involved in cell transformation are likely to be located in this region. As tumor suppressor genes are major targets in neoplastic transformation, we analyzed their expression in these cells. The tumor suppressor Rb is implicated in several cancer types. In our model of MSC transformation, Rb 3,4,5-Trimethoxyphenylacetic acid protein levels are upregulated progressively, and Rb is inactivated by a phosphorylation mechanism in TMC, as described. In addition of Rb, loss of p53 function is common in many tumor types, but this pathway appeared to be functional in our model, as p53 was upregulated and phosphorylated in UV-irradiated cells. We observed higher basal p53 levels in TMC than in MSC, even when they had not been exposed to UV Alprostadil irradiation. In TMC, p16 mRNA and protein were entirely absent, and the Ink4a/Arf locus had been deleted. The increase in basal p53 may thus be due to stabilization by the ubiquitin protein ligase MDM2, due to the lack of p16. Identical results, p16 locus deletion and normal p53 activity, was detected in telomerase-immortalized human MSC.
necessary to transform MSC. We explored DNA repair mechanisms to elucidate their role in MSC transformation. Post-senescent MSC showed downregulation of DNA-PKcs, ERCC3 and Rad51 proteins, each of which is associated to a distinct DNA repair pathway. Extremely restricted clonal selection takes place during cell crisis, and only cells with functional DNA repair mechanisms would continue to grow. TMC have a higher metabolic rate and divide more rapidly than pre- or post-senescence MSC, with a consequent increase in DNA damage. Proteins that participate in DNA repair are upregulated in TMC compared to MSC; this, together with telomere length maintenance, could permit cell survival, despite oxidative damage to DNA and be responsible for TMC karyotype stabilization. Recently it has been published the dependency on oxidative phosphorylation during MSC transformation. We have not detected statistically significant changes of these genes in our microarray experiments, although potential pathways leading to changes in postsenescence MSC and TMC revealed change in stress, toxic events and mitochondrial metabolism pathways. The definitive role of mitochondrial respiration on spontaneous MSC transformation remains to be investigated. A chromosome 5 alteration and a translocation are recurrent, stable features of in vitro cultured TMC. The telomerase gene map to human chromosome 5, suggests that it is activated by internal amplification of this chromosome in TMC. Chromosome 11 alterations are recurrent in tumors. Although we did not detect a target gene in the 3;11 translocation in our model, genes involved in cell transformation are likely to be located in this region. As tumor suppressor genes are major targets in neoplastic transformation, we analyzed their expression in these cells. The tumor suppressor Rb is implicated in several cancer types. In our model of MSC transformation, Rb 3,4,5-Trimethoxyphenylacetic acid protein levels are upregulated progressively, and Rb is inactivated by a phosphorylation mechanism in TMC, as described. In addition of Rb, loss of p53 function is common in many tumor types, but this pathway appeared to be functional in our model, as p53 was upregulated and phosphorylated in UV-irradiated cells. We observed higher basal p53 levels in TMC than in MSC, even when they had not been exposed to UV Alprostadil irradiation. In TMC, p16 mRNA and protein were entirely absent, and the Ink4a/Arf locus had been deleted. The increase in basal p53 may thus be due to stabilization by the ubiquitin protein ligase MDM2, due to the lack of p16. Identical results, p16 locus deletion and normal p53 activity, was detected in telomerase-immortalized human MSC.
In our model during TMC generation these cells acquire a detectable telomerase activity
Leave a reply