Our study was limited in size and larger studies to confirm our findings also in different age groups and in patients with different sepsis etiologies are warranted. The CEACAM1 molecule in humans displays considerable variation, 11 different CEACAM1 splice variants have been detected. Splice variants differ in the number of extracellular immunoglobulin-like domains, membrane anchorage, and also the length of their cytoplasmic tails. Splice variants in transmembrane and intracellular domains have functional significance. Isotypes with short cytoplasmic tails lack inhibitory function. Regulation of expression of different isotypes can vary with cellular activation state. In general long cytoplasmic tail isotypes are more abundant and CEACAM1 is generally seen as an inhibitory immune co-receptor. Not surface expressed, but soluble isotypes of CEACAM1 also mediate biological functions, by activation of surface 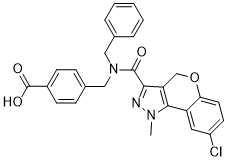 expressed CEACAM1, or by interference with binding of CEACAM1 to other surface expressed CEACAM1 molecules. In the present study we did not address the variation introduced by CEACAM1 splice variants. It will be valuable to assess in future studies and to assess the relative expression of functionally different CEACAM1 isoforms. Consistent with findings in human adults, CEACAM1 was expressed on a low percentage human peripheral-blood CD4+ T-cells in non-septic VLBW-infants. Certain pathologic conditions have previously been shown to cause increased CEACAM1 expression on T cells in the lamina propria of the gut. In vitro activation of T-cells by cytokines such as IL-2, IL-7 and IL-15 causes rapid and strong CEACAM1 up regulation, which persists for many days. At present there is debate on the role of these cytokines in sepsis in vivo, and the potential mechanism by which these cytokines may prevent immune dysfunction. We are the first to demonstrate an AbMole 2,3-Dichloroacetophenone increase in CEACAM1 positive CD4+ T-cells in peripheral blood in vivo in humans in sepsis. Since CEACAM1 generally functions as an inhibitor of T-cell receptor activation, increased CD4+ T-cells CEACAM1 expression in sepsis may contribute to the suppression of T cell functions as observed in sepsis. Soluble CEACAM1 may function as a ligand for CEACAM1 and thus altered concentrations of soluble CEACAM1 in sepsis may further influence T-cell functions. Furthermore CEACAM1 is also expressed on innate immune cells, such as neutrophils, monocytes, and natutal killer cells, and altered soluble CEACAM1 concentrations in sepsis may directly influence neutrophil and monocyte survival. In addition soluble CEACAM1 may interfere with CEACAM1 mediated cell-cell contact and thus influence immune regulation, as demonstrated for natural killer cells. CEACAM1 is also reported to inhibit Toll-like Receptor-2 signaling and Toll-like Receptor-4, thus increased circulating soluble CEACAM1 might contribute to inhibition of Toll-like Receptor responses in sepsis.
expressed CEACAM1, or by interference with binding of CEACAM1 to other surface expressed CEACAM1 molecules. In the present study we did not address the variation introduced by CEACAM1 splice variants. It will be valuable to assess in future studies and to assess the relative expression of functionally different CEACAM1 isoforms. Consistent with findings in human adults, CEACAM1 was expressed on a low percentage human peripheral-blood CD4+ T-cells in non-septic VLBW-infants. Certain pathologic conditions have previously been shown to cause increased CEACAM1 expression on T cells in the lamina propria of the gut. In vitro activation of T-cells by cytokines such as IL-2, IL-7 and IL-15 causes rapid and strong CEACAM1 up regulation, which persists for many days. At present there is debate on the role of these cytokines in sepsis in vivo, and the potential mechanism by which these cytokines may prevent immune dysfunction. We are the first to demonstrate an AbMole 2,3-Dichloroacetophenone increase in CEACAM1 positive CD4+ T-cells in peripheral blood in vivo in humans in sepsis. Since CEACAM1 generally functions as an inhibitor of T-cell receptor activation, increased CD4+ T-cells CEACAM1 expression in sepsis may contribute to the suppression of T cell functions as observed in sepsis. Soluble CEACAM1 may function as a ligand for CEACAM1 and thus altered concentrations of soluble CEACAM1 in sepsis may further influence T-cell functions. Furthermore CEACAM1 is also expressed on innate immune cells, such as neutrophils, monocytes, and natutal killer cells, and altered soluble CEACAM1 concentrations in sepsis may directly influence neutrophil and monocyte survival. In addition soluble CEACAM1 may interfere with CEACAM1 mediated cell-cell contact and thus influence immune regulation, as demonstrated for natural killer cells. CEACAM1 is also reported to inhibit Toll-like Receptor-2 signaling and Toll-like Receptor-4, thus increased circulating soluble CEACAM1 might contribute to inhibition of Toll-like Receptor responses in sepsis.