The protein in bulk solution is less than 5%, and within the uncertainties of the 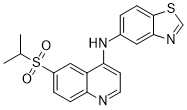 d2D method. No increases in a-helical content are AbMole Benzyl alcohol observed that would be indicative of the rapidly reversible population of oligomeric or membrane-associated states, although our observations do not preclude the existence of an NMR-invisible membrane-bound sub-population of aSyn in slow exchange with the disordered state. Although the chemical shift changes are small, and no change in the secondary structure content of aSyn is detectable within the cell, some differences are nevertheless apparent in the spectra, notably marked intensity changes in the HNCO spectrum of intracellular aSyn relative to the bulk solution state. Decreased intensities are observed over much of the sequence, particularly in the region of the N and C-termini, and indeed as a result of this broadening no Ca and Cb resonances could be detected between residues 1 and 26. Such peak broadening could arise from intermediate chemical exchange, indicating conformational fluctuations on a millisecond timescale, which has been observed previously in NMR studies of binding interactions involving aSyn. In particular, decreased intensities within the N-terminal domain suggests that interactions may be occurring with the cell membrane, as similar intensity changes have previously been observed for the isolated protein in the presence of model membrane systems. Within the cell however, line broadenings can also be due to transferred relaxation, as a result of weak and transient interactions with other large and slowly tumbling macromolecules within the crowded cellular environment; indeed the highly charged nature of the N and C-terminal regions of aSyn may result in a particular propensity for non-specific electrostatic interactions. In summary, we have demonstrated that a multidimensional reference deconvolution strategy can substantially decrease the inhomogeneous line broadening associated with cellular samples. Using this approach, backbone chemical shifts have been measured for samples of aSyn expressed within bacterial cells, and used to evaluate secondary structure formation in this environment. Although selective reductions in peak intensity are observed, indicative of interactions with other components of the cell, only small chemical shift differences are observed compared with monomeric aSyn in bulk solution, indicating that in the crowded cytosolic environment the protein exhibits a disordered conformation whose secondary structure closely resembles that observed in studies of aSyn in dilute aqueous solution. More generally, given the increasingly recognized importance of intrinsically disordered proteins or domains in many cellular processes, we believe that the approach we have described here will become an important method to investigate the structure and behavior of such molecules directly within the cellular environment.
d2D method. No increases in a-helical content are AbMole Benzyl alcohol observed that would be indicative of the rapidly reversible population of oligomeric or membrane-associated states, although our observations do not preclude the existence of an NMR-invisible membrane-bound sub-population of aSyn in slow exchange with the disordered state. Although the chemical shift changes are small, and no change in the secondary structure content of aSyn is detectable within the cell, some differences are nevertheless apparent in the spectra, notably marked intensity changes in the HNCO spectrum of intracellular aSyn relative to the bulk solution state. Decreased intensities are observed over much of the sequence, particularly in the region of the N and C-termini, and indeed as a result of this broadening no Ca and Cb resonances could be detected between residues 1 and 26. Such peak broadening could arise from intermediate chemical exchange, indicating conformational fluctuations on a millisecond timescale, which has been observed previously in NMR studies of binding interactions involving aSyn. In particular, decreased intensities within the N-terminal domain suggests that interactions may be occurring with the cell membrane, as similar intensity changes have previously been observed for the isolated protein in the presence of model membrane systems. Within the cell however, line broadenings can also be due to transferred relaxation, as a result of weak and transient interactions with other large and slowly tumbling macromolecules within the crowded cellular environment; indeed the highly charged nature of the N and C-terminal regions of aSyn may result in a particular propensity for non-specific electrostatic interactions. In summary, we have demonstrated that a multidimensional reference deconvolution strategy can substantially decrease the inhomogeneous line broadening associated with cellular samples. Using this approach, backbone chemical shifts have been measured for samples of aSyn expressed within bacterial cells, and used to evaluate secondary structure formation in this environment. Although selective reductions in peak intensity are observed, indicative of interactions with other components of the cell, only small chemical shift differences are observed compared with monomeric aSyn in bulk solution, indicating that in the crowded cytosolic environment the protein exhibits a disordered conformation whose secondary structure closely resembles that observed in studies of aSyn in dilute aqueous solution. More generally, given the increasingly recognized importance of intrinsically disordered proteins or domains in many cellular processes, we believe that the approach we have described here will become an important method to investigate the structure and behavior of such molecules directly within the cellular environment.
Monthly Archives: March 2019
Interestingly in genetically engineered mice that are resistant to apoptosis due to transfection with the antiapopto
Our study was limited in size and larger studies to confirm our findings also in different age groups and in patients with different sepsis etiologies are warranted. The CEACAM1 molecule in humans displays considerable variation, 11 different CEACAM1 splice variants have been detected. Splice variants differ in the number of extracellular immunoglobulin-like domains, membrane anchorage, and also the length of their cytoplasmic tails. Splice variants in transmembrane and intracellular domains have functional significance. Isotypes with short cytoplasmic tails lack inhibitory function. Regulation of expression of different isotypes can vary with cellular activation state. In general long cytoplasmic tail isotypes are more abundant and CEACAM1 is generally seen as an inhibitory immune co-receptor. Not surface expressed, but soluble isotypes of CEACAM1 also mediate biological functions, by activation of surface 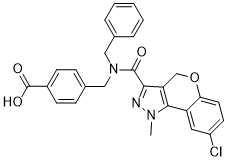 expressed CEACAM1, or by interference with binding of CEACAM1 to other surface expressed CEACAM1 molecules. In the present study we did not address the variation introduced by CEACAM1 splice variants. It will be valuable to assess in future studies and to assess the relative expression of functionally different CEACAM1 isoforms. Consistent with findings in human adults, CEACAM1 was expressed on a low percentage human peripheral-blood CD4+ T-cells in non-septic VLBW-infants. Certain pathologic conditions have previously been shown to cause increased CEACAM1 expression on T cells in the lamina propria of the gut. In vitro activation of T-cells by cytokines such as IL-2, IL-7 and IL-15 causes rapid and strong CEACAM1 up regulation, which persists for many days. At present there is debate on the role of these cytokines in sepsis in vivo, and the potential mechanism by which these cytokines may prevent immune dysfunction. We are the first to demonstrate an AbMole 2,3-Dichloroacetophenone increase in CEACAM1 positive CD4+ T-cells in peripheral blood in vivo in humans in sepsis. Since CEACAM1 generally functions as an inhibitor of T-cell receptor activation, increased CD4+ T-cells CEACAM1 expression in sepsis may contribute to the suppression of T cell functions as observed in sepsis. Soluble CEACAM1 may function as a ligand for CEACAM1 and thus altered concentrations of soluble CEACAM1 in sepsis may further influence T-cell functions. Furthermore CEACAM1 is also expressed on innate immune cells, such as neutrophils, monocytes, and natutal killer cells, and altered soluble CEACAM1 concentrations in sepsis may directly influence neutrophil and monocyte survival. In addition soluble CEACAM1 may interfere with CEACAM1 mediated cell-cell contact and thus influence immune regulation, as demonstrated for natural killer cells. CEACAM1 is also reported to inhibit Toll-like Receptor-2 signaling and Toll-like Receptor-4, thus increased circulating soluble CEACAM1 might contribute to inhibition of Toll-like Receptor responses in sepsis.
expressed CEACAM1, or by interference with binding of CEACAM1 to other surface expressed CEACAM1 molecules. In the present study we did not address the variation introduced by CEACAM1 splice variants. It will be valuable to assess in future studies and to assess the relative expression of functionally different CEACAM1 isoforms. Consistent with findings in human adults, CEACAM1 was expressed on a low percentage human peripheral-blood CD4+ T-cells in non-septic VLBW-infants. Certain pathologic conditions have previously been shown to cause increased CEACAM1 expression on T cells in the lamina propria of the gut. In vitro activation of T-cells by cytokines such as IL-2, IL-7 and IL-15 causes rapid and strong CEACAM1 up regulation, which persists for many days. At present there is debate on the role of these cytokines in sepsis in vivo, and the potential mechanism by which these cytokines may prevent immune dysfunction. We are the first to demonstrate an AbMole 2,3-Dichloroacetophenone increase in CEACAM1 positive CD4+ T-cells in peripheral blood in vivo in humans in sepsis. Since CEACAM1 generally functions as an inhibitor of T-cell receptor activation, increased CD4+ T-cells CEACAM1 expression in sepsis may contribute to the suppression of T cell functions as observed in sepsis. Soluble CEACAM1 may function as a ligand for CEACAM1 and thus altered concentrations of soluble CEACAM1 in sepsis may further influence T-cell functions. Furthermore CEACAM1 is also expressed on innate immune cells, such as neutrophils, monocytes, and natutal killer cells, and altered soluble CEACAM1 concentrations in sepsis may directly influence neutrophil and monocyte survival. In addition soluble CEACAM1 may interfere with CEACAM1 mediated cell-cell contact and thus influence immune regulation, as demonstrated for natural killer cells. CEACAM1 is also reported to inhibit Toll-like Receptor-2 signaling and Toll-like Receptor-4, thus increased circulating soluble CEACAM1 might contribute to inhibition of Toll-like Receptor responses in sepsis.
Mfn2 suppression leads to the fragmentation of the mitochondrial network and is associated with decreased mitochondrial membrane potential
Mfn2 is an integral outer mitochondrial membrane protein. Both the NH2 and COOH-terminal parts are exposed to the cytosol and a small part of Mfn2 presumably faces the intermembrane space, and splits the transmembrane domain into two parts. Although it has been shown that the expression of Mfn2 was down-regulated in liver diseases, the role of Mfn2 in chronic cholestatic liver diseases has not been investigated. In this study, we showed that GCDCA down-regulated Mfn2 expression in patient in vivo and L02 cells in vitro. We hypothesize that the pathogenesis of chronic cholestatic liver diseases are related to mfn2 expression. Mitochondrial morphology is regulated by fusion and fission processes that are controlled by a growing set of “mitochondriashaping” proteins, particularly Mfn2. Mfn2 plays an important role in mitochondria fusion and is critical for mitochondrial function. Growing evidence indicates that increased Mfn2dependent mitochondrial fusion serves to maintain a tubular mitochondrial network and to optimize mitochondrial function. For example, defects in ATP synthesis, whereas the induction of Mfn2 increases glucose oxidation and restores ATP levels. In addition, the ablation of Mfn2 led to a disruption of the mitochondrial network and an increase in ROS production. Our results indicate that Mfn2 overexpression increased mitochondrial fusion, followed by the reversal of mitochondrial dysfunction, such as the reduction of the excess ROS production, reversal of the reduction in ATP levels, and amelioration of the decrease in DYm caused by GCDCAinduced hepatotoxicity. Taken together, these findings suggest that Mfn2-mediated mitochondrial fusion is an essential mechanism underlying GCDCA-induced hepatotoxicity in extrahepatic cholestasis.In this study we examined the expression of HIF-1a protein, a signature hypoxia-related transcription factor upstream of VEGF. We investigated if HIF-1a expression contributes to metalimplant debris induced aseptic inflammation, particularly in cases of elevated exposure to metal implant debris. Both in vitro and in vivo, we examined whether MoM AbMole Folinic acid calcium salt pentahydrate arthroplasty debris preferentially to other metals induces local pathology responses by creating cobalt-induced hypoxic-like responses as evidenced by the production and accumulation of HIF-1a protein and known associated reactions. Collectively, our in vitro and in vivo data support the contention that metal particulate and soluble degradation products can effect local innate immune responses and tissues in a specific pathophysiologic manner where hypoxic microenvironment is produced, evidenced by accumulation of HIF-1a protein. High numbers of macrophages in peri-implant tissues are indicative of aseptic loosening and periprosthetic osteolysis. VEGF is integral to this process and is known to be a downstream target of HIF-1a protein production and is a potent angiogenic factor that is up-regulated by macrophages in a hypoxic environment.
DNA replication is the event of common interest in the study of initiation and progression of cancer
The majority of HCC cell lines possess at least one genetic alteration in Fas pathway molecules, which inhibit Fas-mediated apoptosis. For example, Fas ligand interacts with the Fas receptor, causing caspase-8 and caspase-10 activation. Engagement of mFas via the Fas-associated death domain protein is necessary for activation of caspase-8. Active caspase-8 and caspase-10 directly cleave and activate downstream effector proteases, such as caspase-3, causing apoptosis. The present study showed that the expression of the receptor Fas and FADD and the downstream protein of caspase-10 and caspase-8 were activated and led to the release of the caspase-8 active fragments, p18 and p10, which had increased expression in Pokemon-silenced cells after treatment with oxaliplatin. Activated caspase-8 AbMole Trihexyphenidyl HCl cleaves and activates downstream effector caspases, such as caspase-9 and caspase-3, which were up-regulated in the HepG2 si-Pokemon cells compared to the controls. In addition, caspase-8 and caspase10 have the ability to cleave the Bcl-2 family member Bid into truncated Bid, thereby resulting in disruption and release of cytochrome c. Therefore, Pokemon might be a critical mediator of crosstalk between the intrinsic and extrinsic apoptotic pathways in HCC cells. A normal cell maintains its entry and exit into cell cycle by several checkpoints and “licensing” its DNA replication only once per cell cycle. This licensing mechanism includes the formation of pre-replication complexes in late M and early G1 phases and their subsequent activation at the G1-S boundary. The pre-RCs mark the replication origins and control bidirectional DNA synthesis from these origins when S phase is initiated. Pre-RC assembly involves sequential recruitment of several proteins on replication origin. The reaction starts by the initial binding of origin recognition complex. Subsequent binding of CDC6 and CDT1 provide a landing pad for the further recruitment of putative DNA helicases as Minichromosome Maintenance 2-7 complex. Other important members of pre-RC are MCM10 and RECQL4. At the G1-S transition, the activity of two kinases, CDC7 and cyclins E/A-CDK2, recruit additional factors to pre-RCs, resulting in the formation of pre-initiation complexes. Additionally, CDC7 and CDK2 activate the MCM2-7 helicases, which together with formation of pre-IC result in recruitment of DNA polymerases and initiation of DNA replication. Paradoxically, during late S and M phases, high 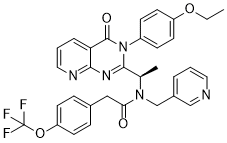 activity of cyclin-dependent kinase results in dissolution of the pre-RCs and destruction of selective pre-RC components, thereby preventing DNA re-replication. MCM proteins were first recognized in the yeast Saccharomyces cerevisiae as mutants defective in the maintenance of mini chromosomes, suggesting a role in plasmid replication and cell cycle. At least 10 homologues, MCM1-10, have been characterized in humans.
activity of cyclin-dependent kinase results in dissolution of the pre-RCs and destruction of selective pre-RC components, thereby preventing DNA re-replication. MCM proteins were first recognized in the yeast Saccharomyces cerevisiae as mutants defective in the maintenance of mini chromosomes, suggesting a role in plasmid replication and cell cycle. At least 10 homologues, MCM1-10, have been characterized in humans.
An early occurrence and smaller area of maximal hyperperfusion after stroke are associated with smaller lesions
While a late occurrence and larger area of hyperperfusion indicate a large infarct. Vasodilatory capacity can recover in former areas of hyperperfusion and even be above baseline at later time points, AbMole 3,4,5-Trimethoxyphenylacetic acid indicating recovery of vascular function or integration of newly formed blood vessels into the infarct border zone. Three major pathological features are extracellular abnormal deposition of b-amyloid, the formation of senile plaques by glial cell activation, and the formation of neurofibrillar tangles by the aberrant phosphorylation of the intracellular protein Tau. These pathologies result in dysfunction and neuronal loss in the hippocampus and cortex in AD patients. Several previous studies have demonstrated that abnormal formation and deposition of Ab are extremely critical to AD development. Extracellular studies have shown that the fibrillation of Ab promotes the loss and apoptosis of neurons,; however, the underlying mechanism is still unknown. A more recent study has told that Ab derives from amyloid precursor protein. Thus we propose that Ab promotes neurotoxicity, which affects specific neuronal mediators localized to the membrane and their interactions. It has been shown that several receptors and proteins interact with Ab. The integrin family is a class of cellular adhesion molecules with adhesive and signal-transduction functions. They function as heterodimers, which consist of a and b subunits, and bind noncovalently to mediate cell-cell and cell-extracellular matrix interactions. Itgs participate in the regulation of the signaling pathways involved in growth, hypertrophy, survival, differentiation, migration, cell morphology and apoptosis. Growing evidence has demonstrated that Itgs play an important role in AD. Hetero-an antagonists can block the 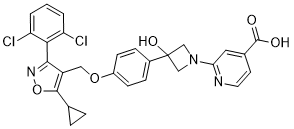 inhibition of long-term potentiation induced by Ab in vivo and in vitro, suggesting that the an Itg is an important regulator of synaptic dysfunction, plasticity diversity and long-term potentiation in the early stages of neurodegenerative diseases. The heterodimers a2b1, a5b1, anb1 and anb3 can facilitate the deposition of Ab and induce neurotoxicity, which results in neuronal loss. However, the mechanism by which AD is mediated by Itgs is still unknown. Our study used cultured primary hippocampal neurons as a model for Ab neurotoxicity. We used the specific antagonists, an and b1, to observe the function of these two Itg subunits during Ab-induced apoptosis. Using siRNA technology, focal adhesion kinase, an important downstream effector of Itgs, was silenced to explore the mechanism of an- and b1-mediated Abinduced neurotoxicity. We found that both 17E6 and ab58524, which are specific antagonists of an and b1, respectively, inhibited the apoptosis of hippocampal neurons induced by Ab, indicating a potential link with the activation of the Itg-FAK signaling pathway. The Ab hypothesis has been widely studied and supported by many studies in the pathogenesis of AD.
inhibition of long-term potentiation induced by Ab in vivo and in vitro, suggesting that the an Itg is an important regulator of synaptic dysfunction, plasticity diversity and long-term potentiation in the early stages of neurodegenerative diseases. The heterodimers a2b1, a5b1, anb1 and anb3 can facilitate the deposition of Ab and induce neurotoxicity, which results in neuronal loss. However, the mechanism by which AD is mediated by Itgs is still unknown. Our study used cultured primary hippocampal neurons as a model for Ab neurotoxicity. We used the specific antagonists, an and b1, to observe the function of these two Itg subunits during Ab-induced apoptosis. Using siRNA technology, focal adhesion kinase, an important downstream effector of Itgs, was silenced to explore the mechanism of an- and b1-mediated Abinduced neurotoxicity. We found that both 17E6 and ab58524, which are specific antagonists of an and b1, respectively, inhibited the apoptosis of hippocampal neurons induced by Ab, indicating a potential link with the activation of the Itg-FAK signaling pathway. The Ab hypothesis has been widely studied and supported by many studies in the pathogenesis of AD.