Since B10 cells express c-kit, the receptor for SCF, CXCR4, the receptor for SDF-1, and VEGFR1, receptor for VEGF, pathways involving SCF/c-kit, SDF-1/ CXCR4 and VEGF/VEGFR are involved in the migration of MSCs to the sites of ICH brain damage and also to corpus callosum and hippocampus. Migration of MSCs toward sites of brain injury may represent an adaptive response of MSCs for the purpose of limiting tissue injury or repair the tissue damage. The mechanism by which the B10 MSCs undergo selective and long distance migration to non-injured sites of corpus callosum and hippocampus might differ from that for the ICH injury and further studies are required to identify the signal for the MSC migration to apparently normal brain region. Brain microenvironment is important in determining survival, migration and differentiation of exogenously transplanted progenitor cells and stem cells. Following the collagenase injection into the striatum, a profuse hemorrhage in the area caused by blood vessels damaged by the proteinase enzyme ensues and the hemorrhage core routinely is absorbed within a week or two, but immune cells released from the vessels remain in the hemorrhage core area. Transplantation of B10 human MSCs is conducted one week after the hemorrhage lesion, thus host immune cells might 4-(Benzyloxy)phenol attack the newly implanted human MSCs in the area. On the other hand, it is known that damaged brain cells and tissues of the host are also capable of releasing molecules that stimulate Amikacin hydrate production of neurotrophic factors in transplanted MSCs. In the present study, no immunosuppressant such as cyclosporine A was utilized to inhibit immune reaction and promote the long-term survival of implanted MSCs in the ICH animals. In earlier studies we have intravenously transplanted immortalized stable human neural stem cells in ICH and focal ischemia model rats without administering immunosuppressant, and found a good survival of grafted NSCs in the brain and a good functional recovery in these animals. However, low survival rate of grafted B10 cells in ICH mice as demonstrated in the present study is a grave concern. The survival rate of transplanted B10 cells at 2 weeks post-transplantation is 41% and that of 6 weeks is 20%. Obvious cause for such poor survival rate of human MSCs in experimental animals is immune-mediated mechanisms by which grafted cells were attacked and destroyed. For that reason, we have to employ immunosuppressants in our future studies to protect grafted human MSCs from cell death in experimental animals. It should be noted that the immunosuppressant cyclosporine has recently been demonstrated to exert neuroprotection in experimental stroke among other disease models. The importance of immunosuppresion in the event of clinical trials using human MSCs in patients suffering from stroke or other  neurological diseases is well recognized. Immunological rejection of neural transplants poses a significant problem to be overcome in order to conduct successfully stem cell-based cell therapy in human patients. A previous study has suggested that the use of progenitors and stem cells for neural grafting is more promising, as these could be maintained in vitro until use, and evoke less immunogenic responses when compared to primary grafts; implantation of immortalized mouse neural stem cells in rat ischemia model has resulted in a good survival at 2 weeks posttransplantation in the absence of immune reaction caused by grafted cells. Further investigations into the specific mechanisms underlying drug actions of immunosuppressants in experimental stroke will certainly improve the therapeutic potential of these drugs for stem cell-based cell therapy.
neurological diseases is well recognized. Immunological rejection of neural transplants poses a significant problem to be overcome in order to conduct successfully stem cell-based cell therapy in human patients. A previous study has suggested that the use of progenitors and stem cells for neural grafting is more promising, as these could be maintained in vitro until use, and evoke less immunogenic responses when compared to primary grafts; implantation of immortalized mouse neural stem cells in rat ischemia model has resulted in a good survival at 2 weeks posttransplantation in the absence of immune reaction caused by grafted cells. Further investigations into the specific mechanisms underlying drug actions of immunosuppressants in experimental stroke will certainly improve the therapeutic potential of these drugs for stem cell-based cell therapy.
Monthly Archives: May 2019
Bladder carcinoma and oropharyngeal cancer where this tumor suppressor is frequently lost
Finally, we propose a two-stage model in which a mesenchymal stem cell becomes a tumor cell. The first step, the senescence bypass or M1 phase, is associated with c-myc overexpression and p16 repression; many DNA repair proteins are subsequently downregulated. Telomere shortening provokes the cell crisis phase, or M2, in which cells undergo stringent selection. TMC then upregulate many DNA repair proteins, which may be necessary for crisis bypass. Finally, escape from crisis is associated with telomere stabilization, Rb hyperphosphorylation and p16 deletion that seems to be Ginsenoside-F5 essential to promote transformation. TMC also upregulate many DNA repair proteins, which may be necessary for crisis bypass. These levels are maintained in TMC and could permit cell survival, despite oxidative damage to DNA. The essential steps in TMC generation described here are basically in agreement with results of other authors working in MSC transformation and these alterations are very similar to molecular changes associated with transformation of other cell types. In epithelial cells, spontaneous immortalization of human keratinocytes exhibited a small number of chromosomal aberrations, reduced p16INK4a mRNA, elevated telomerase activity and functionally normal p53. Immortalization of human prostate cells by cmyc stabilizes telomere length through up-regulation of TERT expression and lack Rb/p16INK4a checkpoint, being easily transformed. In mesodermic cells, fibroblast cell lines immortalized either spontaneously or radio-chemically induced maintaining their telomerase activity, displayed loss of expression of p16INK4a and hyperphosphorylation of Rb. Telomerase-immortalized human fibroblast revealed overexpression of the c-mycand Bmi-1 oncogenes, as well as lossof p14ARF expression, while overexpression of cmyc immortalizes freshly isolated human foreskin fibroblasts displayed a marked decrease in expression of p14ARF. In sum, all these evidences strongly suggest that cells with a mesodermal origin could require a common sequence of oncogenic events to become a tumor cells. How these processes are coordinated or associated with the critical cell evolution/ selection revealed in the culture remains to be studied in deep. In addition, the cause/consequence Lomitapide Mesylate relationship of this molecular signature with the recently characterized mesenchymal to epithelial transition or other potentially involved mechanisms remains also to be determined. Vaccination is a potent and cost-effective counter-measure to the threat of seasonal or pandemic outbreaks of influenza. The influenza virus is among the most devastating viral diseases due to the ease of spread as an aerosol and ability to cause severe sickness and mortality to susceptible humans. Currently licensed seasonal influenza vaccines are only partially protective, particularly in populations at highest risk of severe disease, the very young and the elderly. In addition, there is a need for novel approaches for enhancing immune responses to emerging influenza isolates of avian origin harboring a potential of causing an influenza pandemic outbreak that could infect and kill a considerable number of humans over a short period 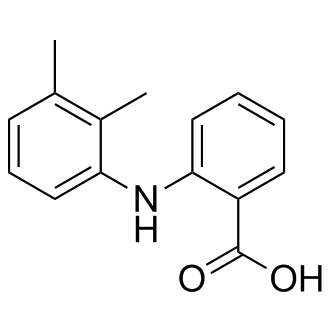 of time. Enhanced immunity is particularly important for vaccines protecting against such emerging strains, since pre-clinical and clinical studies have shown that some of these antigens such as those from H5N1 viruses are less immunogenic than antigens from seasonal influenza subtypes. Recent research, however, has shown improved immunogenicity of some H5N1 antigens if supplemented with proprietary adjuvants. If such approaches are shown to be well-tolerated in humans, they might also be able to stretch the limited supply of currently stockpiled vaccines.
of time. Enhanced immunity is particularly important for vaccines protecting against such emerging strains, since pre-clinical and clinical studies have shown that some of these antigens such as those from H5N1 viruses are less immunogenic than antigens from seasonal influenza subtypes. Recent research, however, has shown improved immunogenicity of some H5N1 antigens if supplemented with proprietary adjuvants. If such approaches are shown to be well-tolerated in humans, they might also be able to stretch the limited supply of currently stockpiled vaccines.
Several approaches are in progress to develop vaccines against H5N1 viruses
To date, products tested in humans have not been effective at producing a strong immune response in a large percentage of subjects tested in clinical trials. However, many of these previous H5N1 vaccine candidates were derived from clade 1 or clade 3 isolates that required multiple doses and/or the use of various adjuvants to achieve levels of antibodies believed to correlate with seroprotection in a majority of subjects tested. Results presented in this report indicate that our A/Indonesia/05/2005 VLP vaccine elicited higher HAI antibody Mepiroxol titers than the A/Viet Nam/1203/2004 VLP vaccine without the use an adjuvant and elicited a robust and broadly reactive immune response following two vaccinations. Interestingly, a single immunization was able to protect mice from virus-induced death, albeit mice administered lower doses of VLPs or rHA had viral replication in the lungs and transient weight loss. These results are similar to recent live attenuated vaccines that required two vaccinations to prevent weight loss, but 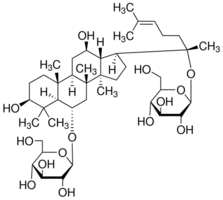 were able to protect ferrets following a single vaccination. The ability to protect humans using a ”one-shot”vaccination regimen is highly desirable for a vaccine against influenza isolates with pandemic potential. Following an outbreak, vaccines that reduce viral titers in the lung and nasal mucosa may slow the transmission of the virus among humans; there may not be sufficient time for a booster shot of vaccine to achieve optimal antibody titers. The VLP vaccine described in this report demonstrates that a one-dose regimen is potentially possible in rodents using a Gomisin-D non-replicating immunogen that can elicit cross-clade protective immune responses and is worthy of evaluation in the clinic. One of the challenges faced by influenza vaccine developers is the ability to protect populations in the face of a spreading pandemic. The next influenza pandemic may be caused by a H5N1 virus and if so, it is not known which clade or subclade will be responsible. Correlates for protection from infection by H5N1 isolates have not been determined. Historically, the HAI assay is the most widely used serological assay for monitoring influenza immunity and is the accepted standard for measuring functional influenza-specific serum antibodies to the hemagglutinin following vaccination. An HAI titer that is greater than 1:40 against a seasonal influenza strain is believed to be protective for,50% of the vaccinated population. However, this does not appear to hold true for avian H5N1 viruses, since no correlation between HAI titer and protective efficacy against H5N1 infection has been reported in animal or human systems. Therefore, new correlates may be necessaryto assess the efficacy of potential H5N1 vaccines. One interesting finding in this study was the correlation between the slower disassociation rates of the VLP-elicited antibody to HA compared to antibodies produced in response to rHA vaccines. In addition, antibodies elicited to the homologous clade 2 rHA had faster association rates compared to antibody binding the heterologous clade 1 rHA. The increase in antibody association ratesinvivo couldbindHAon viruses quickly and perhaps decrease the number of infected cells in the lung, and thus could act to reduce the amount of viral replication to allow the immune system opportunity to better control the infection. In addition, antibodies that are slow to dissociate from the virion may continue to reduce the ability to uncoat and thus restrict the virus post-infection. Further analysis is needed; however, the use of antibody association/dissociation rates may be a more accurate assessment of vaccine efficacy that could potentially correlate with enhanced efficacy.
were able to protect ferrets following a single vaccination. The ability to protect humans using a ”one-shot”vaccination regimen is highly desirable for a vaccine against influenza isolates with pandemic potential. Following an outbreak, vaccines that reduce viral titers in the lung and nasal mucosa may slow the transmission of the virus among humans; there may not be sufficient time for a booster shot of vaccine to achieve optimal antibody titers. The VLP vaccine described in this report demonstrates that a one-dose regimen is potentially possible in rodents using a Gomisin-D non-replicating immunogen that can elicit cross-clade protective immune responses and is worthy of evaluation in the clinic. One of the challenges faced by influenza vaccine developers is the ability to protect populations in the face of a spreading pandemic. The next influenza pandemic may be caused by a H5N1 virus and if so, it is not known which clade or subclade will be responsible. Correlates for protection from infection by H5N1 isolates have not been determined. Historically, the HAI assay is the most widely used serological assay for monitoring influenza immunity and is the accepted standard for measuring functional influenza-specific serum antibodies to the hemagglutinin following vaccination. An HAI titer that is greater than 1:40 against a seasonal influenza strain is believed to be protective for,50% of the vaccinated population. However, this does not appear to hold true for avian H5N1 viruses, since no correlation between HAI titer and protective efficacy against H5N1 infection has been reported in animal or human systems. Therefore, new correlates may be necessaryto assess the efficacy of potential H5N1 vaccines. One interesting finding in this study was the correlation between the slower disassociation rates of the VLP-elicited antibody to HA compared to antibodies produced in response to rHA vaccines. In addition, antibodies elicited to the homologous clade 2 rHA had faster association rates compared to antibody binding the heterologous clade 1 rHA. The increase in antibody association ratesinvivo couldbindHAon viruses quickly and perhaps decrease the number of infected cells in the lung, and thus could act to reduce the amount of viral replication to allow the immune system opportunity to better control the infection. In addition, antibodies that are slow to dissociate from the virion may continue to reduce the ability to uncoat and thus restrict the virus post-infection. Further analysis is needed; however, the use of antibody association/dissociation rates may be a more accurate assessment of vaccine efficacy that could potentially correlate with enhanced efficacy.
In our model during TMC generation these cells acquire a detectable telomerase activity
Telomerase promotes MSC immortalization and, in conjunction with additional events, produces cell transformation. These additional events usually implied an oncogene deregulation. One of the most important oncogenes involved in MSC transformation is c-myc. In our spontaneous model, senescent and post-senescence MSC, as well as TMC, overexpress c-myc. Consistent with our previous results, data from other groups have shown that c-myc seems to be essential to spontaneously transform MSC. In this regard, Funes et al. used retroviral vectors to introduce human telomerase, HVP-16 E6 and E7, H-Ras and SV40 small T antigen, individually or in combination, in human MSC. The combination of TERT, E6, E7 and H-Ras did not induce MSC transformation. Only MSC transduced with ST becomes transformed cells. ST inactivates phosphatase 2A, resulting in c-myc stabilization, suggesting that c-myc might be 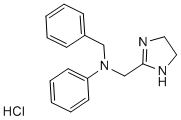 necessary to transform MSC. We explored DNA repair mechanisms to elucidate their role in MSC transformation. Post-senescent MSC showed downregulation of DNA-PKcs, ERCC3 and Rad51 proteins, each of which is associated to a distinct DNA repair pathway. Extremely restricted clonal selection takes place during cell crisis, and only cells with functional DNA repair mechanisms would continue to grow. TMC have a higher metabolic rate and divide more rapidly than pre- or post-senescence MSC, with a consequent increase in DNA damage. Proteins that participate in DNA repair are upregulated in TMC compared to MSC; this, together with telomere length maintenance, could permit cell survival, despite oxidative damage to DNA and be responsible for TMC karyotype stabilization. Recently it has been published the dependency on oxidative phosphorylation during MSC transformation. We have not detected statistically significant changes of these genes in our microarray experiments, although potential pathways leading to changes in postsenescence MSC and TMC revealed change in stress, toxic events and mitochondrial metabolism pathways. The definitive role of mitochondrial respiration on spontaneous MSC transformation remains to be investigated. A chromosome 5 alteration and a translocation are recurrent, stable features of in vitro cultured TMC. The telomerase gene map to human chromosome 5, suggests that it is activated by internal amplification of this chromosome in TMC. Chromosome 11 alterations are recurrent in tumors. Although we did not detect a target gene in the 3;11 translocation in our model, genes involved in cell transformation are likely to be located in this region. As tumor suppressor genes are major targets in neoplastic transformation, we analyzed their expression in these cells. The tumor suppressor Rb is implicated in several cancer types. In our model of MSC transformation, Rb 3,4,5-Trimethoxyphenylacetic acid protein levels are upregulated progressively, and Rb is inactivated by a phosphorylation mechanism in TMC, as described. In addition of Rb, loss of p53 function is common in many tumor types, but this pathway appeared to be functional in our model, as p53 was upregulated and phosphorylated in UV-irradiated cells. We observed higher basal p53 levels in TMC than in MSC, even when they had not been exposed to UV Alprostadil irradiation. In TMC, p16 mRNA and protein were entirely absent, and the Ink4a/Arf locus had been deleted. The increase in basal p53 may thus be due to stabilization by the ubiquitin protein ligase MDM2, due to the lack of p16. Identical results, p16 locus deletion and normal p53 activity, was detected in telomerase-immortalized human MSC.
necessary to transform MSC. We explored DNA repair mechanisms to elucidate their role in MSC transformation. Post-senescent MSC showed downregulation of DNA-PKcs, ERCC3 and Rad51 proteins, each of which is associated to a distinct DNA repair pathway. Extremely restricted clonal selection takes place during cell crisis, and only cells with functional DNA repair mechanisms would continue to grow. TMC have a higher metabolic rate and divide more rapidly than pre- or post-senescence MSC, with a consequent increase in DNA damage. Proteins that participate in DNA repair are upregulated in TMC compared to MSC; this, together with telomere length maintenance, could permit cell survival, despite oxidative damage to DNA and be responsible for TMC karyotype stabilization. Recently it has been published the dependency on oxidative phosphorylation during MSC transformation. We have not detected statistically significant changes of these genes in our microarray experiments, although potential pathways leading to changes in postsenescence MSC and TMC revealed change in stress, toxic events and mitochondrial metabolism pathways. The definitive role of mitochondrial respiration on spontaneous MSC transformation remains to be investigated. A chromosome 5 alteration and a translocation are recurrent, stable features of in vitro cultured TMC. The telomerase gene map to human chromosome 5, suggests that it is activated by internal amplification of this chromosome in TMC. Chromosome 11 alterations are recurrent in tumors. Although we did not detect a target gene in the 3;11 translocation in our model, genes involved in cell transformation are likely to be located in this region. As tumor suppressor genes are major targets in neoplastic transformation, we analyzed their expression in these cells. The tumor suppressor Rb is implicated in several cancer types. In our model of MSC transformation, Rb 3,4,5-Trimethoxyphenylacetic acid protein levels are upregulated progressively, and Rb is inactivated by a phosphorylation mechanism in TMC, as described. In addition of Rb, loss of p53 function is common in many tumor types, but this pathway appeared to be functional in our model, as p53 was upregulated and phosphorylated in UV-irradiated cells. We observed higher basal p53 levels in TMC than in MSC, even when they had not been exposed to UV Alprostadil irradiation. In TMC, p16 mRNA and protein were entirely absent, and the Ink4a/Arf locus had been deleted. The increase in basal p53 may thus be due to stabilization by the ubiquitin protein ligase MDM2, due to the lack of p16. Identical results, p16 locus deletion and normal p53 activity, was detected in telomerase-immortalized human MSC.
An alternative technology for Ab display and selection in the anchored periplasmic
Deletion of PG0717 produced a mutant that lost the capacity to manipulate the host autophagic response and failed to attenuate the production of pro-inflammatory mediators that trigger antimicrobial responses. In addition, perturbed HCAEC responses to coincided with alterations in several putative P. Catharanthine sulfate gingivalis virulence factors including Rgp and Kgp gingipains, Clp protease, and peptidylarginine deiminase. The pleiotropic effects of PG0717 suggest that this protein may be involved in the regulation or processing of multiple virulence properties of P. gingivalis. There are two possibilities to account for the reduction of neural progenitors. These two possibilities are not 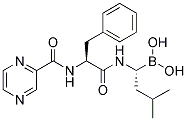 exclusive to each other. One is that ectopic expression of tNolz-1 induces apoptosis of progenitor cells. Indeed, TUNELpositive and activated Amikacin hydrate caspase-3-positive apoptotic cells were found in the germinal zone of the nCT brain. The other possibility is that ectopic expression of tNolz-1 promotes precocious neuronal differentiation, which in turn leads to depletion of progenitor pools. Consistent with this possibility, promotion of cell cycle exit and enhanced ectopic TuJ1 expression was found in the germinal zone of nCT brain. Notably, apoptotic cell clusters containing strong TuJ1-positive signals were found in the germinal zone of SVZ, suggesting that inappropriate premature differentiation of progenitors may cause abnormal cell death in some progenitor cells. In other progenitors, tNolz-1-promoted differentiating cells may survive, because there was a general increase of TuJ1 immunoreactivity in the nCT brain. The transgenic expression of Nolz-1 was driven by the CAG promoter that is likely to have different levels of activity in neural progenitor populations. It is plausible that the expression level of tNolz-1 may be varied in neural progenitors of the nCT brain. A high level of tNolz-1 may promote aberrant differentiation which eventually causes cell death, whereas a low level of tNolz-1 may promote neuronal differentiation of progenitor cells without triggering apoptosis. If so, it implies that the physiological level of Nolz-1 expression must be under rigorous control during neurogenesis. Consistent with this hypothesis, as post-mitotic Nolz1-expressiing neurons migrate from the SVZ to the differentiated MZ, the expression level of Nolz-1 goes through a transition from the high level in the SVZ to the low level in the mantle zone. The expression of antibodies in E. coli, both full-length immunoglobulin G molecules and smaller antigen-binding fragments containing the variable domains from heavy and/or light chains e.g. Fab, single-chain Fv, and single domain Ab, provides a set of powerful technologies for the generation of Abs with novel specificities and improved properties. Current selection of novel therapeutic Abs is based on hybridoma technologies using transgenic mice carrying human Ig genes and screening of Ab gene libraries displayed on the surface of a biological entity. The most common Ab display method is phage display, in which the V-genes are cloned in phagemids as fusions to the minor coat protein III from filamentous bacteriophages of E. coli. The Ab-pIII fusions contain a N-terminal signal peptide to translocate the Ab to the periplasm while the pIII moiety is anchored in the inner membrane. Abs expressed in the periplasm of E. coli generally fold properly due to the presence of protein chaperones and disulfide bond forming and isomerization enzymes. Further, infection of E. coli cells expressing Ab-pIII fusions with a helper bacteriophage allows the production of phage particles displaying the Ab, which can be incubated with the antigen of interest to recover antigen binding clones and amplified by infection of fresh E. coli cells.
exclusive to each other. One is that ectopic expression of tNolz-1 induces apoptosis of progenitor cells. Indeed, TUNELpositive and activated Amikacin hydrate caspase-3-positive apoptotic cells were found in the germinal zone of the nCT brain. The other possibility is that ectopic expression of tNolz-1 promotes precocious neuronal differentiation, which in turn leads to depletion of progenitor pools. Consistent with this possibility, promotion of cell cycle exit and enhanced ectopic TuJ1 expression was found in the germinal zone of nCT brain. Notably, apoptotic cell clusters containing strong TuJ1-positive signals were found in the germinal zone of SVZ, suggesting that inappropriate premature differentiation of progenitors may cause abnormal cell death in some progenitor cells. In other progenitors, tNolz-1-promoted differentiating cells may survive, because there was a general increase of TuJ1 immunoreactivity in the nCT brain. The transgenic expression of Nolz-1 was driven by the CAG promoter that is likely to have different levels of activity in neural progenitor populations. It is plausible that the expression level of tNolz-1 may be varied in neural progenitors of the nCT brain. A high level of tNolz-1 may promote aberrant differentiation which eventually causes cell death, whereas a low level of tNolz-1 may promote neuronal differentiation of progenitor cells without triggering apoptosis. If so, it implies that the physiological level of Nolz-1 expression must be under rigorous control during neurogenesis. Consistent with this hypothesis, as post-mitotic Nolz1-expressiing neurons migrate from the SVZ to the differentiated MZ, the expression level of Nolz-1 goes through a transition from the high level in the SVZ to the low level in the mantle zone. The expression of antibodies in E. coli, both full-length immunoglobulin G molecules and smaller antigen-binding fragments containing the variable domains from heavy and/or light chains e.g. Fab, single-chain Fv, and single domain Ab, provides a set of powerful technologies for the generation of Abs with novel specificities and improved properties. Current selection of novel therapeutic Abs is based on hybridoma technologies using transgenic mice carrying human Ig genes and screening of Ab gene libraries displayed on the surface of a biological entity. The most common Ab display method is phage display, in which the V-genes are cloned in phagemids as fusions to the minor coat protein III from filamentous bacteriophages of E. coli. The Ab-pIII fusions contain a N-terminal signal peptide to translocate the Ab to the periplasm while the pIII moiety is anchored in the inner membrane. Abs expressed in the periplasm of E. coli generally fold properly due to the presence of protein chaperones and disulfide bond forming and isomerization enzymes. Further, infection of E. coli cells expressing Ab-pIII fusions with a helper bacteriophage allows the production of phage particles displaying the Ab, which can be incubated with the antigen of interest to recover antigen binding clones and amplified by infection of fresh E. coli cells.