The genetics of mice that model human FTD, and has shown the capacity to model pre-disease, genotype-specific phenotypes. By using mice expressing either of two distinct transgenes as the source of SCs, we have established that neurosphere Albaspidin-AA cultures maintain genotype-specific characteristics. Our results lend credence 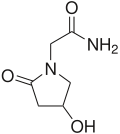 to the growing body of data supporting the development and use of patient specific-stem cell lines to study disease. We have already shown that these cells reproducibly mimic biological events of the mice from which they were derived, and that they express the appropriate molecules involved in tau modification genetically validating the utility of SC as a model system. We are now in a position to interrogate the system. We are focusing on microarray analysis experiments to uncover differentially regulated genes between tauP301L and tauwt mice and neurosphere cultures. Preliminary results are encouraging and show consistency in genotype-specific gene expression patterns among independently derived neurosphere lines. This will direct hypotheses about potential pathways targeted in cells that carry the tauP301L gene. By extending this research to patientspecific SCs, high-throughput cell based genetic screening assays could uncover small molecules and potential pathways involved in pathogenesis and, ideally, the genetic specificity of the system may lead to treatment therapies tailored to unique patient needs. As part of the development of the central nervous system, neuronal cells are required to coordinately expand their population and integrate a functional network by connecting through growing dendrites and axons, cell prolongations that are collectively denominated neurites. Neurite outgrowth is widely employed as a typical marker for assessing differentiation in cultured neuronal cells such as PC12 rat pheochromocitoma cells and Neuro-2a mouse neuroblastoma cells. Although the mechanisms by which neuronal cells control the timing of cell proliferation and differentiation are still poorly understood, animal and cellbased studies have shown that a number of extrinsic factors, including growth factors and cytokines, such as epidermal growth factor, platelet-derived growth factor, and brain-derived neurotrophic factor, have crucial influence on the functional fate of neuronal cells. The binding of these factors to plasma membrane receptors triggers the activation of central signal transduction cascades, including MAPK, Akt and Src, which will initiate the transcriptional program Folinic acid calcium salt pentahydrate needed for neuronal differentiation. In addition to the aforementioned neurotrophic factors, Wnt proteins, a family of secreted proteins that modulates a myriad of cellular and organismal functions, including cellular proliferation, axis formation and organogenesis, are key regulators of neuronal differentiation. Binding of Wnt ligands to their receptor complex, consisting of members of Frizzled and lowdensity lipoprotein family LRP5 and LRP6, activates two main cascades of intracellular signals, the canonical b-catenin/TCF pathway, and the less understood non-canonical Wnt signaling which is independent of b-catenin. This signaling includes the planar cell polarity-convergent extension pathway, via Jun N-terminal kinase, Rho and Rac mediators, and the Wnt/Calcium pathway which signals through Dvl to induce calcium influx and the activation of protein kinase C and calcium/calmodulin-dependent protein kinase II. Wnt proteins have been shown to participate in the mechanisms of cell replication and differentiation in neurons.
to the growing body of data supporting the development and use of patient specific-stem cell lines to study disease. We have already shown that these cells reproducibly mimic biological events of the mice from which they were derived, and that they express the appropriate molecules involved in tau modification genetically validating the utility of SC as a model system. We are now in a position to interrogate the system. We are focusing on microarray analysis experiments to uncover differentially regulated genes between tauP301L and tauwt mice and neurosphere cultures. Preliminary results are encouraging and show consistency in genotype-specific gene expression patterns among independently derived neurosphere lines. This will direct hypotheses about potential pathways targeted in cells that carry the tauP301L gene. By extending this research to patientspecific SCs, high-throughput cell based genetic screening assays could uncover small molecules and potential pathways involved in pathogenesis and, ideally, the genetic specificity of the system may lead to treatment therapies tailored to unique patient needs. As part of the development of the central nervous system, neuronal cells are required to coordinately expand their population and integrate a functional network by connecting through growing dendrites and axons, cell prolongations that are collectively denominated neurites. Neurite outgrowth is widely employed as a typical marker for assessing differentiation in cultured neuronal cells such as PC12 rat pheochromocitoma cells and Neuro-2a mouse neuroblastoma cells. Although the mechanisms by which neuronal cells control the timing of cell proliferation and differentiation are still poorly understood, animal and cellbased studies have shown that a number of extrinsic factors, including growth factors and cytokines, such as epidermal growth factor, platelet-derived growth factor, and brain-derived neurotrophic factor, have crucial influence on the functional fate of neuronal cells. The binding of these factors to plasma membrane receptors triggers the activation of central signal transduction cascades, including MAPK, Akt and Src, which will initiate the transcriptional program Folinic acid calcium salt pentahydrate needed for neuronal differentiation. In addition to the aforementioned neurotrophic factors, Wnt proteins, a family of secreted proteins that modulates a myriad of cellular and organismal functions, including cellular proliferation, axis formation and organogenesis, are key regulators of neuronal differentiation. Binding of Wnt ligands to their receptor complex, consisting of members of Frizzled and lowdensity lipoprotein family LRP5 and LRP6, activates two main cascades of intracellular signals, the canonical b-catenin/TCF pathway, and the less understood non-canonical Wnt signaling which is independent of b-catenin. This signaling includes the planar cell polarity-convergent extension pathway, via Jun N-terminal kinase, Rho and Rac mediators, and the Wnt/Calcium pathway which signals through Dvl to induce calcium influx and the activation of protein kinase C and calcium/calmodulin-dependent protein kinase II. Wnt proteins have been shown to participate in the mechanisms of cell replication and differentiation in neurons.
By activating both signaling branches of Wnt pathways but the mechanisms by signals regulate the timing of these processes
Leave a reply