Partly be explained by tolerance development and polarization differences in TLR-receptor distribution. PRNP mRNA was significantly up-regulated in TNBScolitis. This was verified by ISH which showed expression in submucosal immune cells and some expression in epithelial cells in animals not yet exposed to TNBS, but expression was more pronounced after colitis induction both in the submucosal immune cells and within the epithelium. PrPc is expressed in several tissues, Mepiroxol including neural tissue, gut-associated lymphoid tissue, enteroendocrine cells and enterocytes. Its function is not well known. Expression of PrPc in the host is thought to be necessary for the propagation and transmission of prion infection. In addition, both pro- and anti-inflammatory roles have been suggested. Reduced inflammatory infiltration has been seen in PrPc-deficient mice upon stimulation with TLR2 and TLR4 ligands. In contrast, PrPc over-expressing mice are resistant to colitis induction with DSS while PrPc deficient mice had significantly higher levels of inflammatory cytokines compared to DSS-treated wild type mice. Increased intestinal barrier permeability has been demonstrated in PrPc-deficient mice, Folinic acid calcium salt pentahydrate further supporting a protecting role for PrPc in colitis and IBD. PPARc is one of three identified isoforms of peroxisome proliferator-activated receptors and is expressed in several tissues; expression is high in the colonic epithelial cells. It is thought to have an important role in modulation of inflammation. PPARc suppresses inflammatory cytokines like TNFa, interleukin6 and IL-1b and maintains defensin production. Gene expression analysis revealed down-regulation of PPARc after colitis induction. In endoscopic biopsies evaluated by ISH, a high basal expression of PPARc in non-inflamed colonic epithelium, while a considerable down-regulation in inflamed colon epithelium was identified. This was accompanied by downregulation of FABP1. FABP1 transcription, in the microarray was confirmed by PCR. The intracellular level of FABP1 positively regulates PPARc and the two proteins also have a direct proteinprotein interaction. Regulation of FABPs is seen in IBD, PPARc is down-regulated in UC and gene polymorphisms of PPARc have been implicated in the pathogenesis of CD. PPARc-agonists have been shown to ameliorate TNBS-induced colitis and the PPARc ligand rosiglitazone has shown efficacy in mild to moderately active IBD. PPARc might also inhibit tumor growth in colorectal cancer. Alternatively, FABP1 has been suggested as a biomarker of CD because a 10fold up-regulation was found in one study. Our findings, in contrast, are consistent with loss of PPARc-mediated antiinflammatory effects. This supports a potentially important function for PPARc in the regulation of colonic inflammation and indicates that FABPs could also be a possible target for therapy. Among the 20 genes with highest FC at all time points were SPINK4; lipopolysaccharide binding protein, ADA and RETNLB. PCR validation was performed for SPINK4 and ADA. SPINK4 has recently been identified as a risk locus for UC and increased expression is seen in untreated celiac disease. LBP transfers LPS to the LPS-signaling receptor complex, promotes innate immunity against gram negative bacteria, and may serve as a marker of disease activity in CD. ADA 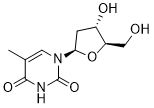 deactivates adenosine and thus diminishes the potentially protective effects of adenosine in mucosal inflammation and hypoxia. A beneficial effect after inhibition of ADA has been demonstrated in experimental colitis. RETNLB had a FC of 470 and 253 at T7 and T12, respectively.
deactivates adenosine and thus diminishes the potentially protective effects of adenosine in mucosal inflammation and hypoxia. A beneficial effect after inhibition of ADA has been demonstrated in experimental colitis. RETNLB had a FC of 470 and 253 at T7 and T12, respectively.
RETNLBdeficiency increases intestinal permeability and RETNLB seems immune response
Leave a reply