These compounds represent new scaffolds for future rhomboid inhibitor and ABP development. In the last decade, small molecule ABPs have substantially impacted protease research, with applications ranging from activity profiling to target discovery and fluorescent imaging. ABPs have also facilitated HTS for ill-characterized enzymes using fluorescent polarization. This HTS has been executed on soluble, but not on membrane enzymes. Recent reports of the first ABPs for intramembrane proteases from the rhomboid family have therefore urged us to investigate FluoPol ABPP for use with membrane enzymes. We have managed this by employing a low concentration of a mild detergent and also found that the surfactant Pluronic F-127 is essential for a good signal-to-noise ratio, probably by facilitating the solubilization of the fluorescent dye. Overall, this resulted in an HTS compatible assay with a high Z-value of 0.9. We are confident that the assay will enable the screening of other poorly characterized membrane-anchored or membrane embedded enzymes. The screening of rhomboids from different organisms is subject of our future research efforts. The special advantage of FluoPol ABPP is that it does not require a substrate, but uses a broad-spectrum ABP. For rhomboids, no small molecule fluorogenic or chromogenic substrates are available as for soluble proteases. One FRET-based polypeptide has been used for screening, but this cannot be used universally. Protein substrates are still the standard assay technique to monitor rhomboid activity. However, the detection of cleavage of these substrates is laborious. Hence, the development and optimization of fluorescent ABPs for rhomboids and other membrane enzymes will likely assist inhibitor discovery for such enzymes. Since the discovery of rhomboids as intramembrane proteases in 2001, inhibitor development has gained momentum slowly. FP-R, for example, reacts with 82% of all mouse metabolic serine hydrolases, which makes it an excellent broad-spectrum ABP. The rhomboid inhibitors based on 4-chloro-isocoumarins have gone through several optimization steps, from the weakly inhibiting DCI, to JLK-6 and S016, which is currently the most potent isocoumarin inhibitor for the E. coli rhomboid GlpG. Still, S016 is more potent against chymotrypsin than against GlpG. The b-lactone scaffold that we have found here, is structurally related to b-lactams. b-lactones are more reactive than b-lactams, and unsurprisingly, b-lactams only act as rhomboid inhibitors when activated with a N-sulfonyl group. The b-lactones 31 and 43 are less potent than the 4chloro-isocoumarin S016, but they have a higher XL-184 potency against GlpG than against trypsin and chymotrypsin. Hence, b-lactones may have the potential to be more selective inhibitors than 4chloro-isocoumarins. Although compounds 31 and 43 also target other  serine hydrolases, the b-lactone scaffold can be readily influenced in its selectivity by changing the substituents on the lactone ring. Compound 43 for example, is an acylated form of 44, the natural product vibralactone. Vibralactone is inactive against rhomboid, probably due to the presence of a polar hydroxyl group that may result in unfavourable interactions with the hydrophobic rhomboid TMDs. When this hydroxyl group is blocked as an ester function in compound 43, it yields an active inhibitor. These structures illustrate the possibility to optimize the b-lactone scaffold for usage against rhomboids. We have shown that the b-lactones covalently and irreversibly react with the active site serine of GlpG. This makes them well suitable for use as ‘warheads’ for ABPs. Compounds 31 and 43 contain an alkyne group in their structure, amenable to click chemistry-mediated derivatization. This feature allowed the direct on-gel visualization of the active rhomboid form. Hence, this study adds two new ABPs to the rhomboid chemical toolbox. Since blactones have already been successfully used for ABPP of serine hydrolases in lysates and live bacterial cells, we expect them to be useful tools for the in vivo functional study of bacterial rhomboids. VE-821 Influenza A viruses infect a wide range of avian and mammalian hosts. The worldwide spread of avian flu as well as the subsequent outbreak of the 2009 H1N1 flu has raised public concerns of the global influenza pandemics due to the high morbidity and mortality.
serine hydrolases, the b-lactone scaffold can be readily influenced in its selectivity by changing the substituents on the lactone ring. Compound 43 for example, is an acylated form of 44, the natural product vibralactone. Vibralactone is inactive against rhomboid, probably due to the presence of a polar hydroxyl group that may result in unfavourable interactions with the hydrophobic rhomboid TMDs. When this hydroxyl group is blocked as an ester function in compound 43, it yields an active inhibitor. These structures illustrate the possibility to optimize the b-lactone scaffold for usage against rhomboids. We have shown that the b-lactones covalently and irreversibly react with the active site serine of GlpG. This makes them well suitable for use as ‘warheads’ for ABPs. Compounds 31 and 43 contain an alkyne group in their structure, amenable to click chemistry-mediated derivatization. This feature allowed the direct on-gel visualization of the active rhomboid form. Hence, this study adds two new ABPs to the rhomboid chemical toolbox. Since blactones have already been successfully used for ABPP of serine hydrolases in lysates and live bacterial cells, we expect them to be useful tools for the in vivo functional study of bacterial rhomboids. VE-821 Influenza A viruses infect a wide range of avian and mammalian hosts. The worldwide spread of avian flu as well as the subsequent outbreak of the 2009 H1N1 flu has raised public concerns of the global influenza pandemics due to the high morbidity and mortality.
Monthly Archives: July 2019
This hypothesis has recently been supported by the discovery of a scorpion toxin containing the DDH motif
The gene sequences of several other peptides, predicted to contain ICK motifs, and present in the Cucurbitaceae family, have been determined recently. These precursor proteins have similar architectures to MCh-1 and MCh-2, with the mature peptides at the terminal region of the precursor proteins. However, the cleavage sites that yield the mature peptide vary for these different disulfide-rich peptides. Known cleavage sites include after an alanine residue for a trypsin inhibitor TI-I from Trichosanthes kirilowii and cleavage after a glycine residue to yield TGTI-II from the towel gourd, as shown in Figure 6. Both MCh-1 and MCh-2 appear to require cleavage after a leucine residue to yield the mature peptides. The diversity in the cleavage sites suggests that a range of proteases are involved in the maturation of plant ICK peptides. The oxidative folding of MCh-1 was analyzed using RP-HPLC and MS. MCh-1 represents an ideal peptide for investigating the oxidative SJN 2511 ALK inhibitor Refolding process of the ICK motif since its in vitro oxidation is slow enough to allow the isolation and characterization of intermediates formed during folding. Although IIa was the major intermediate, numerous other intermediates were present in the oxidative refolding of MCh-1, both in the presence and in the absence of the shuffling reagent glutathione. Refolding of purified IIa resulted in numerous species, including two-disulfide and three-disulfide isomers. This complexity in the folding pathway indicates that IIa does not convert directly to the native form. By contrast with the oxidative refolding process, the reductive unfolding is very simple and IIa was the only intermediate observed. It is interesting to note the differences in retention times of the intermediates during the selective reduction and the stepwise alkylation, or the oxidative refolding and the stepwise alkylation. The intermediate IIa eluted just before the native peptide and the similarity of the retention times of the intermediate with that of the native peptide indicates that the three-dimensional structures are similar and that the intermediate also contains a compact structure. The retention time of NEM-alkylated IIa was almost the same as that of IIa. This similarity in retention times suggests that the NEM replacement did not affect the conformation of IIa. By contrast, the retention times of NEM-alkylated Ia, the fully alkylated MCh-1 and intermediate Ia decreased dramatically relative to the native peptide, which indicates that those structures are not compact. Different folds of MCh-1 intermediates are shown in Figure 7. The two-disulfide intermediate observed during the folding of MCh-1 is equivalent to the major intermediates previously reported for the plant ICK peptides EETI-II, MCoTI-II and kalata B1. The structures of these intermediates have been analyzed using NMR spectroscopy and shown to contain the native fold but lack the CysI-CysIV disulfide bond. EETI-II and MCoTI-II are both squash trypsin inhibitors with similar sequences, and do not share sequence similarity with kalata B1, with the exception of the six cysteine residues. However, both MCoTI-II and kalata B1 contain a cyclic backbone in addition to the ICK motif. Therefore this intermediate accumulates during folding in both cyclic and acyclic peptides and does not appear to have stringent sequence requirements given the diversity of sequences across these different peptides. Despite the conservation of the intermediate IIa in the folding of various ICK peptides, the pathways involved in the formation of the native peptide vary. The intermediate IIa observed during the folding of MCoTI-II appears to be the direct precursor to the native peptide, in contrast to the intermediate observed during the folding of kalata B1, which requires rearrangement of the disulfide bonds to form the native peptide. The MCh-1 IIa intermediate is also Gefitinib likely to require rearrangement of the disulfide bonds based on the additional intermediates observed in the analysis of the folding of purified intermediate IIa. The conservation of the two-disulfide intermediate implies an integral role in the folding of the ICK motif. Indeed, it is tempting to speculate that this intermediate is involved with the evolution of the ICK given it is equivalent to the proposed ancestral fold. The disulfide-directed b-hairpin comprising two-disulfide bonds equivalent to the CysII-CysV and CysIII-CysVI bonds in the ICK motif has been proposed to be the ancestral .gif) fold of the ICK.
fold of the ICK.
Previously DN59 had been shown to be non-toxic to cultured cells by some treated particles
This difference might be caused by the use of more than 1,000 times more virus in the genome degradation experiments, or having only partially released genomes after incubation with DN59. Although particles with partially released genomes are likely to be non-infectious, their genomes may still have been protected from degradation by RNase. This would cause the IC50 for the genome degradation assay to shift upwards in concentration compared to the FFU reduction assay. The separation of the genome from the virus particle would be expected to irreversibly destroy infectivity. Reversibility was tested directly by treating virus with peptide at a concentration expected to produce approximately 80% inhibition of infectivity, then diluting the virus:peptide mixture 10 fold to a peptide concentration expected to produce negligible inhibition. No reversibility of inhibition was observed in these experiments. The release of the virus RNA genome was confirmed by centrifuging peptide-treated, untreated, and triton detergenttreated virus particles BKM120 through a tartrate density gradient, and monitoring the amount of RNA genome and E protein in each fraction. The results showed that the genome and E protein comigrate in intact virus particles, but migrate to different fractions following peptide or detergent treatment, indicating that the genome and E protein are no longer associated after peptide treatment. To Rapamycin confirm that there were no other targets for the inhibitory activity of DN59, time of addition and infectivity assays in a different target cell line were conducted. There was no inhibition of infectivity when mammalian target cells were incubated with DN59 and then washed prior to the addition of virus. Nor was there inhibition of infectivity when DN59 was added after the cells had been infected. Furthermore, after coincubation of virus with DN59, infection was inhibited in both mammalian epithelial and mosquito cells, showing that changes of the host cell type and corresponding viral entry pathway did not result in changes of the neutralization profile. Therefore, it can be concluded that DN59 acts directly on the virus particle to release the RNA genome rather than on some other viral or cellular target. Based on these experiments, DN59 appears to induce formation of holes in the viral membrane. Thus, DN59 might be expected to interact with lipid membranes and form holes or otherwise disrupt membrane bilayer structures. Consistent with this expectation, a concentration-dependent increase in the fluorescence of the tryptophan residue at peptide position 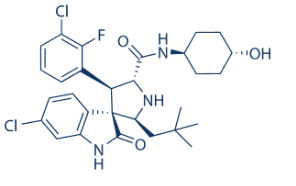 nine was observed when peptide was mixed with liposome vesicles composed of either 1palmitoyl-2-oleoyl-phosphatidylcholine, or a 9:1 molar ratio of POPC and 1-palmitoyl-2-oleoyl-phosphatidylglycerol, indicative of strong binding. Also, addition of DN59 peptide to either POPC or POPC/POPG vesicles containing a fluorescent dye and quencher caused extensive disruption of membrane integrity and leakage of contents to occur at concentrations as low as 2 mM. These observations confirm that DN59 interacts strongly with liposome vesicles and is capable of disrupting artificial lipid bilayers. The observed peptide-lipid membrane interactions are not merely charge based, as binding and disruption occurred with both zwitterionic POPC vesicles as well as negatively-charged 9:1 POPC/POPG vesicles. Supporting these observations, a recent study of the membrane disruption ability of overlapping peptides from dengue virus type 2 C and E proteins showed that E protein stem derived peptides were highly disruptive to liposomes prepared with a wide variety of lipid compositions.
nine was observed when peptide was mixed with liposome vesicles composed of either 1palmitoyl-2-oleoyl-phosphatidylcholine, or a 9:1 molar ratio of POPC and 1-palmitoyl-2-oleoyl-phosphatidylglycerol, indicative of strong binding. Also, addition of DN59 peptide to either POPC or POPC/POPG vesicles containing a fluorescent dye and quencher caused extensive disruption of membrane integrity and leakage of contents to occur at concentrations as low as 2 mM. These observations confirm that DN59 interacts strongly with liposome vesicles and is capable of disrupting artificial lipid bilayers. The observed peptide-lipid membrane interactions are not merely charge based, as binding and disruption occurred with both zwitterionic POPC vesicles as well as negatively-charged 9:1 POPC/POPG vesicles. Supporting these observations, a recent study of the membrane disruption ability of overlapping peptides from dengue virus type 2 C and E proteins showed that E protein stem derived peptides were highly disruptive to liposomes prepared with a wide variety of lipid compositions.
Biochemical experiments indicate that EzrA interacts directly with FtsZ to inhibit assembly
Thus, like Insv, Insb appears to SJN 2511 function in a partly redundant manner with H. Additionally, while loss of insb and insv activities similarly enhanced the H haplo-insufficient Masitinib VEGFR/PDGFR inhibitor phenotype, no genetic interaction was observed in double mutant flies. One possible interpretation for this lack of genetic interaction is that Insv and Insb act together to regulate the same process, so that the complete loss of one or both genes have similar 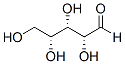 phenotypic consequences. Since Insv did not regulate the expression of insb, one possibility is that Insb positively regulates the expression of the insv gene and that Insv antagonizes Notch. Alternatively, the two proteins may act together to repress the expression of Notch target genes via the Su binding sites. Consistent with this, Insv was proposed to repress the expression of Notch target genes by two mechanisms: first in a Su-dependent mechanims, Insv would act as a CSL co-repressor to promote repression through Su binding sites; second, Insv may directly bind DNA via its BEN domain and regulate gene expression in a Su-independent manner. Whether Insb physically interacts with Insv and regulates its transcriptional activities await biochemical studies. While a functional homolog of Insv has recently been characterized in the mouse, no clear homolog of Insb could be easily identified in vertebrates. Thus, deciphering how Insb regulates in flies the activities of Insv and other CSL associated co-repressors, such as H, may provide new insights into molecular mechanisms of co-repression by CSL-associated factors. Finally, while the expression and function of Insb was primarily studied here in the context of sensory organ development, this gene was also expressed at high levels in neuroblasts of the developing larval brain, suggesting that Insb may have a broader role as a Notch antagonist. In conclusion, our study identified Insb as a nuclear SOP/ neuron-specific antagonist of Notch signaling that may act together with Insv to repress the expression of Notch target genes. Assembly of the highly conserved tubulin-like protein FtsZ into a ring structure at the nascent division site initiates the process of cell division in most bacteria. The FtsZ ring serves as a foundation for assembly of the division machinery and constricts at the leading edge of the invaginating septum during cytokinesis. The precise temporal and spatial regulation of cell division is achieved through the actions of a host of proteins, which interact directly with FtsZ to modulate assembly of the cytokinetic ring. Some of these modulators help stabilize FtsZ polymers at midcell and thus maintain the integrity of the cytokinetic ring. In both Bacillus subtilis and Escherichia coli, the location of FtsZ ring formation appears to be dictated in part through the actions of proteins that inhibit FtsZ assembly at aberrant subcellular positions. In B. subtilis, EzrA, a 65 kDa membrane bound protein, plays an important role in both modulatory roles. EzrA is among the first set of proteins to localize to the cytokinetic ring. Null mutations in ezrA reduce the critical concentration of FtsZ required for ring formation in vivo and result in the formation of extra FtsZ rings and septa at cell poles. In contrast to loss of function mutations in other positional regulators of bacterial cell division, the loss of EzrA significantly increases the stability of the medial FtsZ ring, rendering it resistant to overexpression of division inhibitors. Null mutations in ezrA or a point mutation that disrupts EzrA localization to midcell increase cell length by more than 50%, consistent with a model in which EzrA is required for the efficient use of the medial division site.
phenotypic consequences. Since Insv did not regulate the expression of insb, one possibility is that Insb positively regulates the expression of the insv gene and that Insv antagonizes Notch. Alternatively, the two proteins may act together to repress the expression of Notch target genes via the Su binding sites. Consistent with this, Insv was proposed to repress the expression of Notch target genes by two mechanisms: first in a Su-dependent mechanims, Insv would act as a CSL co-repressor to promote repression through Su binding sites; second, Insv may directly bind DNA via its BEN domain and regulate gene expression in a Su-independent manner. Whether Insb physically interacts with Insv and regulates its transcriptional activities await biochemical studies. While a functional homolog of Insv has recently been characterized in the mouse, no clear homolog of Insb could be easily identified in vertebrates. Thus, deciphering how Insb regulates in flies the activities of Insv and other CSL associated co-repressors, such as H, may provide new insights into molecular mechanisms of co-repression by CSL-associated factors. Finally, while the expression and function of Insb was primarily studied here in the context of sensory organ development, this gene was also expressed at high levels in neuroblasts of the developing larval brain, suggesting that Insb may have a broader role as a Notch antagonist. In conclusion, our study identified Insb as a nuclear SOP/ neuron-specific antagonist of Notch signaling that may act together with Insv to repress the expression of Notch target genes. Assembly of the highly conserved tubulin-like protein FtsZ into a ring structure at the nascent division site initiates the process of cell division in most bacteria. The FtsZ ring serves as a foundation for assembly of the division machinery and constricts at the leading edge of the invaginating septum during cytokinesis. The precise temporal and spatial regulation of cell division is achieved through the actions of a host of proteins, which interact directly with FtsZ to modulate assembly of the cytokinetic ring. Some of these modulators help stabilize FtsZ polymers at midcell and thus maintain the integrity of the cytokinetic ring. In both Bacillus subtilis and Escherichia coli, the location of FtsZ ring formation appears to be dictated in part through the actions of proteins that inhibit FtsZ assembly at aberrant subcellular positions. In B. subtilis, EzrA, a 65 kDa membrane bound protein, plays an important role in both modulatory roles. EzrA is among the first set of proteins to localize to the cytokinetic ring. Null mutations in ezrA reduce the critical concentration of FtsZ required for ring formation in vivo and result in the formation of extra FtsZ rings and septa at cell poles. In contrast to loss of function mutations in other positional regulators of bacterial cell division, the loss of EzrA significantly increases the stability of the medial FtsZ ring, rendering it resistant to overexpression of division inhibitors. Null mutations in ezrA or a point mutation that disrupts EzrA localization to midcell increase cell length by more than 50%, consistent with a model in which EzrA is required for the efficient use of the medial division site.
It should be more difficult to unbind NHI than other inhibitors known to bind to the same site
Likewise, it should be most difficult to unbind FX11 if the binding models from conventional MD simulations represent its experimental binding modes. The LY2157299 TGF-beta inhibitor pulling force as a function of pulling distance was plotted, and the work required to pull the inhibitor out of the binding site was also calculated by integration. Pulling Asite binders turned out to be much easier than S-site binders in spite of their comparable binding affinities. This is probably caused by the need to dissociate more interactions and overcome more steric clashes when pulling S-site binders, especially 2B4 and NHI, whose binding kept the mobile loop closed. To demonstrate the influence of different initial loop conformations on the pulling of S-site binders, 6P3 was pulled from two different representative structures, one with the mobile loop open and the other closed. As expected, starting from the open conformation required much smaller peak force and less work than starting from the closed conformation. Conversely, pulling 2B4 from two slightly different representative structures, both of which have the mobile loop closed, resulted in a similar peak force and almost identical amount of work. Thus, both the site of binding and the initial conformation of the mobile loop can affect the difficulty of unbinding LDHA inhibitors. Regardless of the loop conformation, it took less work and smaller peak force to dissociate 6P3 than 2B4, suggesting that 2B4 is indeed a stronger binder than 6P3. More importantly, the work performed to unbind NHI is much less than that of 2B4 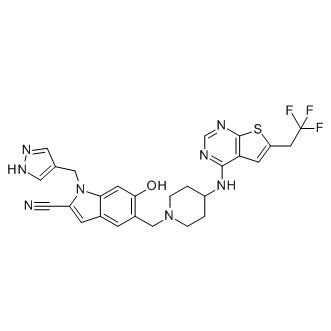 and 6P3 when pulling from the loop-closed conformation, contradicting their relative experimental binding affinities. This suggests that the S-site is not the preferred binding site for NHI. The dissociation of FX11, whose binding kept the mobile loop open during conventional MD simulations, turned out to be more difficult than 6P3 when starting from the loop-open conformation. Thus, it appeared that FX11 could bind within the S-site and is indeed a stronger inhibitor than 6P3. Yet, it should be noted that their initial loop conformations are different. The mobile loop in LDHA:FX11S complex is “more closed” than that in LDHA:6P3, and it should be more difficult to unbind FX11 than 6P3 even if they have similar binding affinities within the S-site. The initial loop conformation had a similar impact on the pulling of both dual-site inhibitors. With the mobile loop being initially closed, the pulling of 0SN required more work and larger peak force than that of 1E4, even though 0SN is a slightly weaker inhibitor. Additionally, the work spent on pulling dualsite inhibitors is larger than the combined values of their single-site counterparts, indicating that the linker moiety in both dual-site inhibitors contributes to their binding. The use of a tetrameric model to study LDHA computationally has been attempted previously.However, those studies were based on evidence from either geometry optimization or short-term MD simulations with restraints to prevent large conformational changes.In contrast, the present study employed moderate-length MD simulations with sufficient Fingolimod system sizeand no restraints to approximate physiological conditions, further justifying the use of the tetrameric form in such computational studies. Of note, LDHAs from different speciesmight show different dynamics. However, we restricted this study to human LDHA, which is most relevant to the development of anticancer agents; only 0SN has been cocrystalized with human LDHA among the ligands studied.
and 6P3 when pulling from the loop-closed conformation, contradicting their relative experimental binding affinities. This suggests that the S-site is not the preferred binding site for NHI. The dissociation of FX11, whose binding kept the mobile loop open during conventional MD simulations, turned out to be more difficult than 6P3 when starting from the loop-open conformation. Thus, it appeared that FX11 could bind within the S-site and is indeed a stronger inhibitor than 6P3. Yet, it should be noted that their initial loop conformations are different. The mobile loop in LDHA:FX11S complex is “more closed” than that in LDHA:6P3, and it should be more difficult to unbind FX11 than 6P3 even if they have similar binding affinities within the S-site. The initial loop conformation had a similar impact on the pulling of both dual-site inhibitors. With the mobile loop being initially closed, the pulling of 0SN required more work and larger peak force than that of 1E4, even though 0SN is a slightly weaker inhibitor. Additionally, the work spent on pulling dualsite inhibitors is larger than the combined values of their single-site counterparts, indicating that the linker moiety in both dual-site inhibitors contributes to their binding. The use of a tetrameric model to study LDHA computationally has been attempted previously.However, those studies were based on evidence from either geometry optimization or short-term MD simulations with restraints to prevent large conformational changes.In contrast, the present study employed moderate-length MD simulations with sufficient Fingolimod system sizeand no restraints to approximate physiological conditions, further justifying the use of the tetrameric form in such computational studies. Of note, LDHAs from different speciesmight show different dynamics. However, we restricted this study to human LDHA, which is most relevant to the development of anticancer agents; only 0SN has been cocrystalized with human LDHA among the ligands studied.