Indeed, recent studies that aimed to decipher the biological mechanisms involved in the development and maintenance of chronic aggression have consistently found a key role for the serotonin pathway, suggesting lower serotoninergic activity associated with FDA-approved Compound Library aggression/impulsivity. Tryptophan Hydroxylase is the rate-limiting enzyme in the synthesis of serotonin. In humans, Perez-Rodriguez et al. tested several polymorphisms of TPH2 and found an extended haplotype associated with enhanced aggressiveness, suicidal behavior, and susceptibility to borderline personality disorder. The glucocorticoid receptor, whose epigenetic changes, were associated with early-life stress, plays also a key role in HPA axis related stress reactivity and aggressive behavior in pigs. The stress response and the HPA axis have also been associated with aggression. Both hyper- and hypo-active HPA axis are associated with aggression in humans. It is perhaps not surprising that these genes are differentially methylated only in women where the HPA negative feedback control is known to be more sensitive than in males. The same is true for rodents where it has been shown that female rodents have higher HPA axis activity under basal and stressinduced conditions than males. As previously observed in men, several of the genes in the female aggression signature play roles in cytokine function and inflammatory response. Recent studies have shown that cytokines are associated with various behavioral disorders such as anxiety, depression, suicide, FG-4592 childhood mood disorder and post-traumatic stress disorder. It was also suggested that cytokines might play a role in the neurobiology of aggression since they are expressed in brain regions already known to be involved in aggression and behavior. Our results show that IL1RN belongs to both the male and female aggression signatures. Moreover, cytokines expression in blood and gene methylation in T cells was recently found to associate with men CPA in the same subjects as the one studied here. Further work is needed to investigate the role of peripheral cytokines in aggression but taken together these data suggest that cytokine regulation could play an important role in human behavior and mental health. It is noteworthy that although these genes are clearly involved in brain function, we also observed changes in DNA methylation in T cells associated with aggressive behavior. Similar results were obtained when we recently compared rhesus monkey DNA methylation changes in prefrontal cortex and T cells in response to differences in maternal rearing. This analysis revealed both tissue specific alterations as well as common differentially methylated regions in T cells and prefrontal cortex. Two previous reports suggested that brain function-specific genes were differentially methylated in peripheral blood cells in association with physical aggression. Further work is needed to understand why seemingly brain-specific genes would be differentially methylated in blood DNA in association with aggression and other environmental factors. There are three important limitations to this study. First, although we recruited subjects from a large longitudinal study, we managed to assess only a small number of women with chronic physical aggression because they represent a very small percentage of the population and are difficult to recruit. Thus, replications are needed from similar longitudinal studies to confirm our results.
Monthly Archives: July 2019
Clearly further work is needed to fully appreciate its function on malignant transformation of stem cells
We further compared the significance of differentially expressed proteins in our early and later passage hESCs in an optimized culture condition since we wished to exclude the influence of other changes associated with the in vitro culture adaptation. Our data showed relatively stable expression of HDAC2 in long-term in vitro cultures of normal hESCs, but displayed increasing levels during tumorigenesis followed with increased histone deacetylation. These observations supported the idea that enhanced HDAC2 expression may be associated with malignant transformation by regulating the architecture of chromatin. Thus HDAC2 could serve as a potential marker for abnormal hESCs with a tendency of initiating progression to the malignant state. Also, our data showed increased levels of proteins associated with DNA methylation, including DNMT3A, DNMT3B, AZD6244 DNMT1 and KPNB1. These proteins might contribute to the increased methylation of CpG islands and silencing of affected target genes, which are frequently found in human cancer. Though DNMT3B increases in karyotypically abnormal hESCs, in much the way as HDAC2, expression of DNMT3B was also enhanced in long-term in vitro and optimal culture condition, which was consistent with the gene mRNA expression levels. Recent studies have also shown that DNA methyltransferases, and DNMT3B, were correlated with HDAC1 and HDAC2 and were involved in the epigenetic regulation by silencing transcription and promoting cell proliferation and tumorigenesis. DNMT3B can also interact with HDACs 1/2 and other components of the epigenetic machinery to establish the chromatin environment. Peter Andrews reported that hESCs might undergo culture adaptation in long-term in vitro culture and that variations in gene expression might reflect the aberrant karyotype of the cells or might result from karyotypically silent epigenetic changes, implying that adaptation reflects an alteration in the balance between self-renewal and differentiation. Longterm in vitro cultured hESCs show a high risk of genomic instability due to culture conditions. Thus, we optimized our culture system including the preparation of feeder cells by irradiation, control of the density of feeder cells and passage by manual cutting. In optimized conditions,BAY-60-7550 hESCs show a stable normal karyotype, even after more than two years of in vitro culture. The increased expression of DNMT3B in culture suggested that it might be relevant to culture adaptation and reflect the progressive adaptation of self-renewing cells to their culture conditions. However, the possibility of tumorigenesis can not be excluded, and further research is required in future work to empirically demonstrate or disprove this. Moreover, aberrant expression of CTNNB1, which is the epigenetically modified protein, induces malignant pathways in normal cells and abnormal activity of CTNNB1 also exists in malignant progression. In stem cells, expression of CTNNB1 might serve as a multifunctional protein with a central role in stem cell renewal and differentiation. Recent studies have shown that CTNNB1 could regulate Tert expression through the interaction with Klf4, and thereby telomere length, which could be critical in human cancer. Here, enhanced expression of CTNNB1 and increased expression target genes accompanied with more serious transformation in hESCs implied its role in stem cell-derived tumor initiation and progression.
Represented by multiple replicates may be considered a surrogate for a different clinical state
In so doing, it describes analytical methods that can be applied to identify variability arising from technical sources across the workflow, from sample procurement through LC-MS. It also compares two approaches to quantify proteins from peptide data. Further, it presents baseline statistical data for 81 relatively abundant CSF proteins within a neurologically normal group of older individuals, calculates the technical variability and gradient effects observed in the measurement of these proteins within multiple replicates of a pooled CSF sample, and illustrates how selective subsets of these proteins might be used to classify samples that differ by biological phenotype. Thus, it provides a framework for future experiments that will evaluate CSF samples from individuals with neurological diseases, in search of relevant biomarkers. Recently, other reports have also described the use of this technique for CSF proteome characterization and biomarker discovery. Impressively, some have identified and quantified hundreds or thousands of proteins in a single CSF sample, by referencing the unique LC retention times and m/z values of the extracted ion chromatograms of peptides to an annotated ‘library’ of retention times and m/z values compiled from previous MS/MS analyses of similar CSF preparations. For this current experiment, no such annotated AMT library was available. Instead, MS1 and MS2 R428 scanning were performed simultaneously to enable the annotation of peptides in real time. This approach availed the identification of a comparatively smaller number of proteins, but was wholly adequate for the purpose of this experiment, which was not intended to discover novel 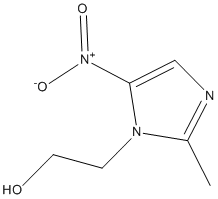 rare CSF proteins or to maximize the number of proteins identified. As it happens, recent instrumentation advances during the short interval since this experiment was conducted now allow for the annotation of many more peptides in real time, effectively increasing the sensitivity of simultaneous scanning; these changes have reduced the popularity of the more laborious AMT approach. Regardless, even without such advances, either approach is likely to identify promising candidate biomarkers. Indeed, even in this limited experiment, in which tandem mass spectrometry was triggered solely on the basis of relatively high peptide abundance, a comparatively modest list of 81 proteins generated sufficient diagnostic potential to allow perfect segregation of ‘individual’ and ‘pooled’ sample replicates with a much smaller subset of 24 selected proteins. It is also encouraging that many of the 81 proteins have already been reported as potential biomarkers for AD by multiple independent groups. Indeed, because none of these previously reported candidate biomarkers have been vetted sufficiently to be applied in clinical trials, they will have to be studied further: individually and in combination; in larger cohorts and in different diseases. Thus, particularly with recent advances, this technique is well suited for application in future AD research studies to facilitate the U0126 validation of promising biomarkers. A final point of discussion addresses the purpose and the implications of the hierarchical clustering analyses performed in this study. Such analyses are employed here to illustrate the potential of this technique to measure ensembles of proteins that can classify samples according to desired characteristics. In most biomarker discovery studies, such clustering analyses would be preceded by a selection process in which candidate biomarkers are vetted on the basis of statistical association with a diagnosis of interest. In the current study, because the samples analyzed do not strictly represent two different disease states, the proteins were evaluated, instead, for their ability to segregate CSF from different sources.
rare CSF proteins or to maximize the number of proteins identified. As it happens, recent instrumentation advances during the short interval since this experiment was conducted now allow for the annotation of many more peptides in real time, effectively increasing the sensitivity of simultaneous scanning; these changes have reduced the popularity of the more laborious AMT approach. Regardless, even without such advances, either approach is likely to identify promising candidate biomarkers. Indeed, even in this limited experiment, in which tandem mass spectrometry was triggered solely on the basis of relatively high peptide abundance, a comparatively modest list of 81 proteins generated sufficient diagnostic potential to allow perfect segregation of ‘individual’ and ‘pooled’ sample replicates with a much smaller subset of 24 selected proteins. It is also encouraging that many of the 81 proteins have already been reported as potential biomarkers for AD by multiple independent groups. Indeed, because none of these previously reported candidate biomarkers have been vetted sufficiently to be applied in clinical trials, they will have to be studied further: individually and in combination; in larger cohorts and in different diseases. Thus, particularly with recent advances, this technique is well suited for application in future AD research studies to facilitate the U0126 validation of promising biomarkers. A final point of discussion addresses the purpose and the implications of the hierarchical clustering analyses performed in this study. Such analyses are employed here to illustrate the potential of this technique to measure ensembles of proteins that can classify samples according to desired characteristics. In most biomarker discovery studies, such clustering analyses would be preceded by a selection process in which candidate biomarkers are vetted on the basis of statistical association with a diagnosis of interest. In the current study, because the samples analyzed do not strictly represent two different disease states, the proteins were evaluated, instead, for their ability to segregate CSF from different sources.
The actual sequences and genes of origin of the peptides responsible for XICs of interest can be determined by LC-MS
Statistically significant differences between sample groups. Given sufficient sample and analytical time, extensive annotated libraries that match XICs to their unique peptide sequences can be accumulated for a given sample type. This accurate mass and time tag approach can be applied retrospectively or prospectively, reducing or eliminating the need for tandem mass spectrometry in subsequent studies of that biofluid within a given laboratory. Alternatively, MS2 can be performed in series with LC-MS during primary quantitative data acquisition. In this work, we apply quantitative label-free LC-MS/MS to the analysis of replicate CSF samples: to identify sources of technical variability that can be mitigated; to assess the inter-individual and residual technical variance with which this technique measures numerous proteins in cognitively normal subject samples; to compare 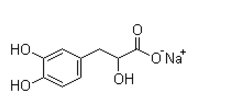 alternative strategies for protein quantification from quantitative peptide data; and to test the ability of its output to segregate biological samples according to desired biomarker characteristics. In this way, we demonstrate the suitability of quantitative label-free LC-MS/MS as a tool for CSF biomarker discovery. During the performance of each of these LC-MS analyses, the mass spectrometer was operating in data dependent mode and automatically isolated the most abundant ions for MS2. After processing, the MS2 data were then searched against the UNIPROT database for annotation as described in Fig. S3. The annotated features were processed using a visual script that was executed with Rosetta ElucidatorTM software. The annotated peak intensities were normalized as described in Fig. S3. Because some peptides were detected in more than one charge state, the individual charge states for each peptide were combined, yielding 1360 annotated isotope groups. Additionally, when multiple isotope groups within an LC-MS analysis were found to be associated with a common peptide sequence, their peak intensities were summed, yielding 926 annotated, aligned peptide ion chromatograms across the 25 samples. The data from each step of the sequential processing diagrammed in Fig. 2A were exported into a spread sheet, grouped as gene names and imported into DAnTE-R software for further analysis. To evaluate variability in the proteomics workflow at the level of annotated peptides, non-clustering heat maps of Axitinib Pearson correlation coefficients were used. PCC values were generated using the log2 transformed peptide intensity data for all pair-wise sample comparisons. Fig. 2B shows the symmetrical matrix of PCC values from all pairwise correlations among the pooled and individual sample aliquots, calculated from all annotated charge groups; for the purpose of comparison, Fig. S11 shows a similar matrix, calculated from all aligned charge groups. The diagonal white squares represent self-comparisons that yield a perfect correlation of 1.0 on a scale of 0.65 to 1.0; as shown by the color bar, imperfect correlations are AbMole BioScience kinase inhibitors represented by increased shading that ranges from yellow to orange to red, with the lowest values appearing as black squares. These Pearson correlation heat maps corroborate the ion current results; pairwise comparisons of the pooled sample with the lowest ion current yielded uniformly low PCC’s, represented by intense black bars in Fig. 2B and Fig. S11, with samples P13b and P5 showing the poorest correlation. The corresponding P13 vs. P5 scatter plot appears as a wide, homogeneous cloud of log2 transformed aligned charge group intensities. In contrast, the scatter plot of two pooled samples with a high PCC, represented in Fig. 2D, appears predominantly as a tight linear cluster. We concluded from these data that TIC values below, 25% of the mean value of the data set are not normalized by the algorithm described in Materials and Methods.
alternative strategies for protein quantification from quantitative peptide data; and to test the ability of its output to segregate biological samples according to desired biomarker characteristics. In this way, we demonstrate the suitability of quantitative label-free LC-MS/MS as a tool for CSF biomarker discovery. During the performance of each of these LC-MS analyses, the mass spectrometer was operating in data dependent mode and automatically isolated the most abundant ions for MS2. After processing, the MS2 data were then searched against the UNIPROT database for annotation as described in Fig. S3. The annotated features were processed using a visual script that was executed with Rosetta ElucidatorTM software. The annotated peak intensities were normalized as described in Fig. S3. Because some peptides were detected in more than one charge state, the individual charge states for each peptide were combined, yielding 1360 annotated isotope groups. Additionally, when multiple isotope groups within an LC-MS analysis were found to be associated with a common peptide sequence, their peak intensities were summed, yielding 926 annotated, aligned peptide ion chromatograms across the 25 samples. The data from each step of the sequential processing diagrammed in Fig. 2A were exported into a spread sheet, grouped as gene names and imported into DAnTE-R software for further analysis. To evaluate variability in the proteomics workflow at the level of annotated peptides, non-clustering heat maps of Axitinib Pearson correlation coefficients were used. PCC values were generated using the log2 transformed peptide intensity data for all pair-wise sample comparisons. Fig. 2B shows the symmetrical matrix of PCC values from all pairwise correlations among the pooled and individual sample aliquots, calculated from all annotated charge groups; for the purpose of comparison, Fig. S11 shows a similar matrix, calculated from all aligned charge groups. The diagonal white squares represent self-comparisons that yield a perfect correlation of 1.0 on a scale of 0.65 to 1.0; as shown by the color bar, imperfect correlations are AbMole BioScience kinase inhibitors represented by increased shading that ranges from yellow to orange to red, with the lowest values appearing as black squares. These Pearson correlation heat maps corroborate the ion current results; pairwise comparisons of the pooled sample with the lowest ion current yielded uniformly low PCC’s, represented by intense black bars in Fig. 2B and Fig. S11, with samples P13b and P5 showing the poorest correlation. The corresponding P13 vs. P5 scatter plot appears as a wide, homogeneous cloud of log2 transformed aligned charge group intensities. In contrast, the scatter plot of two pooled samples with a high PCC, represented in Fig. 2D, appears predominantly as a tight linear cluster. We concluded from these data that TIC values below, 25% of the mean value of the data set are not normalized by the algorithm described in Materials and Methods.
Thus the signals of XICs with identical retention/elution times and values can be directly compared
Indeed, the failure of many recent clinical trials aimed at AD is commonly attributed to the exclusive enrollment of participants who already have mild or moderate dementia and concomitant neuron loss. Therefore, tools and strategies must be developed to diagnose and enroll individuals in the SCH772984 942183-80-4 pre-clinical phase of AD, when brain pathology is present but cognition remains intact. By definition, this phase is not reliably detected by clinical examination, so biomarkers will be required for diagnosis. Ideally, biomarkers should also estimate an individual��s risk of impending cognitive decline and even allow monitoring of pathological progression and response to treatment. Once such biomarkers are developed, clinical trials should become more efficient and effective treatments will be identified more quickly. Subsequently, once successful treatments are identified, these biomarkers are likely to remain useful in a clinical setting. Some progress has already been made in this direction. To date, leading modalities for such biomarkers include radiological imaging and cerebrospinal fluid analysis. Both techniques can detect amyloid deposits in the brain either directly, using amyloidbinding tracer compounds and positron emission tomography, or indirectly, by measuring low CSF beta-amyloid42 concentrations that correlate with amyloid deposition. Imaging and fluid biomarker studies have also shown potential to predict cognitive decline by measuring amyloid deposition, regional volumetric and metabolic changes in the brain, or specific changes in the CSF proteome. CSF SAR131675 analysis may even allow classification of disease stage and monitoring of acute changes in response to disease modifying therapies, as illustrated recently with gamma-secretase inhibitors. In spite of these advances, however, these techniques must still be improved. Additional biomarkers will be required to improve the sensitivity and specificity of pre-clinical AD diagnosis, increase the accuracy of prognosis, and expand the breadth of pathophysiological changes that can be monitored. CSF proteome analysis provides a favorable arena for such efforts. Indeed, many increasingly more powerful yet complementary proteomics technologies have been leveled at CSF biomarker discovery in the past decade, including: variations of 2D gel electrophoresis; SELDI-TOF-MS; offline LC �C MALDI-TOF; and LC-MS/MS with either isotopecoded affinity tags, Tandem Mass Tags or iTRAQ. These techniques have all been used successfully to identify candidate biomarkers because they provide accurate relative quantitative information between or among samples. In order to provide this information, they share a common requirement: proteins or peptides must be stained or labeled for precise and accurate quantification. This requirement necessarily increases procedural costs and also may introduce additional sources of error. These techniques also share a major limitation in clinical proteomics : they cannot readily be used to compare directly large 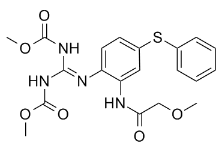 numbers of samples, even with advances in multiplexing technologies and strategies. This second shortcoming is quite important because most CSF biomarkers, at least in the AD field, show relatively modest disease-associated quantitative changes, on the order of 30%; detecting such differences with statistical rigor in a cross-sectional study requires precise and accurate measurements with potentially hundreds of CSF samples. Label-free, quantitative proteomic methods have emerged that obviate the requirement for protein staining or peptide labeling. Many of these ��label-free�� approaches take advantage of the correlation between high-resolution LC/MS extracted ion currents and peptide abundances. Bioinformatics software tools have been developed that align LC elution times and accurate m/z values of the XIC��s across numerous samples.
numbers of samples, even with advances in multiplexing technologies and strategies. This second shortcoming is quite important because most CSF biomarkers, at least in the AD field, show relatively modest disease-associated quantitative changes, on the order of 30%; detecting such differences with statistical rigor in a cross-sectional study requires precise and accurate measurements with potentially hundreds of CSF samples. Label-free, quantitative proteomic methods have emerged that obviate the requirement for protein staining or peptide labeling. Many of these ��label-free�� approaches take advantage of the correlation between high-resolution LC/MS extracted ion currents and peptide abundances. Bioinformatics software tools have been developed that align LC elution times and accurate m/z values of the XIC��s across numerous samples.