Ghrelin is a 28-amino acid peptide, produced by the stomach and hypothalamic neurons. It is the endogenous ligand of the growth hormone secretagogue receptor 1a. Ghrelin receptors are expressed by various brain regions, such as the arcuate nucleus, lateral hypothalamus, VMH and suprachiasmatic nucleus, Evofosfamide inquirer structures known to be involved in feeding and sleep regulation. Ghrelin secretion is stimulated by fasting and ghrelin enhances feeding and increases adiposity in rats. Growing body of evidence indicates that ghrelin signaling plays a role in the function of arousal mechanisms. Systemic, intracerebroventricular or intrahypothalamic administration of ghrelin suppresses sleep in rats. Ghrelin receptor KO mice show attenuated arousal responses to food deprivation and to the exposure of novel  environment. Ghrelin is also implicated in the function of thermoregulatory mechanisms and in the integration of sleep and thermoregulatory responses. Central administration of ghrelin diminishes the activity of brown adipose tissue, a key effector organ in non-shivering thermogenesis, by suppressing the activity of its sympathetic innervation. The product of the preproghrelin gene play a role in coordinating thermoregulatory/metabolic and sleep responses to metabolic challenges. When fasted in the cold, normal mice develop hypothermic bouts and increased sleep during these hypothermic periods. Ghrelin deficient preproghrelin knockout mice are incapable of mounting sleep responses under these conditions and enter precipitous, lethal, hypothermia. FAS inhibitors, such as C75 greatly suppress ghrelin production by the stomach and the hypothalamus. C75 potently suppresses eating and energy expenditure. Since ghrelin stimulates feeding and transgenic mice with elevated circulating ghrelin levels have increased energy expenditure, it seemed possible that the inhibitory effects of C75 on feeding and energy expenditure are mediated by its suppressive action on ghrelin production. To test this hypothesis, we determined the effects of C75 on feeding, metabolism, sleep and motor activity in ghrelin receptor deficient mice. Our major finding is that systemic injection of C75 suppresses motor activity, REMS, and SWA of the EEG in both normal and ghrelin receptor KO mice. These behavioral and sleep effects are accompanied by decreases in VO2, body temperature and RER. We confirm our and others’ previous findings that spontaneous sleep-wake activity, motor activity and food intake on standard laboratory diet are not affected in ghrelin receptor KO mice. Our results also confirm that C75 elicits robust dose-dependent inhibition of 24-h food intake. The effects of C75 on the daily rhythm of feeding have not been reported before. We show that C75 abolished the diurnal rhythm of feeding. Night-time food intake was decreased to the level normally seen during the day, the rest period in mice. The effects of C75 on motor activity have not been quantified previously. In two studies, no “gross Nilotinib changes” in the activity of mice or no “obvious motoric effects” in rats were observed after systemic injection of C75. In the present study, we quantified the effects of C75 on spontaneous motor activity in two separate sets of experiments, by using two different methods. We found that spontaneous motor activity was decreased after ip injection of C75 as measured both by the movement of implanted transponders over a horizontal receiver in Experiment 1 and by the interruption of infrared beams in Experiment 2. The mechanism of the anorectic effects of C75 is not well understood. It is possible that C75 stimulates satiety circuits and/ or suppresses orexigenic mechanisms or inhibits feeding by eliciting visceral illness.
environment. Ghrelin is also implicated in the function of thermoregulatory mechanisms and in the integration of sleep and thermoregulatory responses. Central administration of ghrelin diminishes the activity of brown adipose tissue, a key effector organ in non-shivering thermogenesis, by suppressing the activity of its sympathetic innervation. The product of the preproghrelin gene play a role in coordinating thermoregulatory/metabolic and sleep responses to metabolic challenges. When fasted in the cold, normal mice develop hypothermic bouts and increased sleep during these hypothermic periods. Ghrelin deficient preproghrelin knockout mice are incapable of mounting sleep responses under these conditions and enter precipitous, lethal, hypothermia. FAS inhibitors, such as C75 greatly suppress ghrelin production by the stomach and the hypothalamus. C75 potently suppresses eating and energy expenditure. Since ghrelin stimulates feeding and transgenic mice with elevated circulating ghrelin levels have increased energy expenditure, it seemed possible that the inhibitory effects of C75 on feeding and energy expenditure are mediated by its suppressive action on ghrelin production. To test this hypothesis, we determined the effects of C75 on feeding, metabolism, sleep and motor activity in ghrelin receptor deficient mice. Our major finding is that systemic injection of C75 suppresses motor activity, REMS, and SWA of the EEG in both normal and ghrelin receptor KO mice. These behavioral and sleep effects are accompanied by decreases in VO2, body temperature and RER. We confirm our and others’ previous findings that spontaneous sleep-wake activity, motor activity and food intake on standard laboratory diet are not affected in ghrelin receptor KO mice. Our results also confirm that C75 elicits robust dose-dependent inhibition of 24-h food intake. The effects of C75 on the daily rhythm of feeding have not been reported before. We show that C75 abolished the diurnal rhythm of feeding. Night-time food intake was decreased to the level normally seen during the day, the rest period in mice. The effects of C75 on motor activity have not been quantified previously. In two studies, no “gross Nilotinib changes” in the activity of mice or no “obvious motoric effects” in rats were observed after systemic injection of C75. In the present study, we quantified the effects of C75 on spontaneous motor activity in two separate sets of experiments, by using two different methods. We found that spontaneous motor activity was decreased after ip injection of C75 as measured both by the movement of implanted transponders over a horizontal receiver in Experiment 1 and by the interruption of infrared beams in Experiment 2. The mechanism of the anorectic effects of C75 is not well understood. It is possible that C75 stimulates satiety circuits and/ or suppresses orexigenic mechanisms or inhibits feeding by eliciting visceral illness.
Monthly Archives: August 2019
These sleep and behavioral effects are strikingly similar to those we found in response to C75 treatment
Ghrelin plays a central role in physiologic orexigenic mechanisms and stimulates motor activity. It has been postulated that increased behavioral activity and feeding in the beginning of the dark period are due to the activation of ghrelin circuits in the brain of nocturnal rodents. C75 is known to inhibit ghrelin secretion in mice thus it seemed possible that suppressed ghrelin secretion may account for both the anorectic and motor activity-suppressing effects of C75. In this case, intact ghrelin receptor-mediated signaling would be required for the manifestations of these actions, i.e., C75 would not affect eating and activity in animals that lack ghrelin receptors. The finding that C75-induced anorexia and motoric inhibition are not attenuated in ghrelin 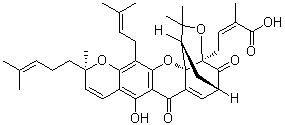 receptor KO mice indicates that these effects of C75 are unrelated to the function of ghrelin receptors. We found significant correlation between the anorectic and motor activity effects of C75. An overall decrease in motor activity may, in theory, lead to decreased feeding. Although causality cannot be determined with certainty in the present study, it seems unlikely that the anorectic effects are exclusively due to the general CHIR-99021 suppression of motor activity. First, while the overall time-courses correlate significantly, there is a meaningful dissociation between the motor activity- and feeding-suppressive effects of C75. For example, in the ghrelin receptor KO mice, motor activity was unchanged in the first 4 h after C75 Evofosfamide treatment but feeding was already suppressed to,20% of the baseline. Second, if it is the decreased motor activity that interferes with normal feeding behavior after C75 injection then one would expect that the drive for food, i.e., hunger, progressively increases during the dark. Hunger induced by food restriction is accompanied by characteristic changes in sleep-wake activity and c-Fos activation pattern in the brain. Overnight food deprivation results in a,24% increase in waking time during the dark in mice. This arousal response was not observed after C75 injection supporting the notion that the animals were not hungry, in spite of the fact that they ate less than 18% of their normal food amount during the dark period. Also, food deprivation stimulates c-Fos expression in orexigenic brain structures such as the paraventricular nucleus, ARC and LH, but systemic C75 treatment fails to elicit similar activation pattern. A possible explanation for the decreased feeding after C75 injection is that C75 elicits a satiety-like state. The sleep findings, however, do not support this notion. Both naturally occurring satiety that follows feeding as well as injection of satietyinducing hormones such as cholecystokinin lead to increases in sleep. In our study, however, C75 induced dosedependent and long-lasting suppression of REMS. Thus the sleep phenotype after C75 treatment does not match fasting or satiated conditions but shows close similarity to the sleep pattern described in visceral pain models. Visceral illness elicited by LiCl injections is accompanied by transient increase in wakefulness followed by long-lasting suppression of REMS. An ip bolus injection of LiCl causes significant increase in the latency and a significant reduction in the occurrence of REM sleep in the immediate hours following the injection. In contrast, NREM sleep occurrence is only slightly affected by lithium administration. LiCl treatment significantly reduces the relative delta power of the EEG after LiCl treatment. We also observed the suppression of EEG SWA, i.e. delta waves, after C75 administration. Furthermore, LiCl treatment leads to behavioral inactivity and causes rats to lie quietly on the floor of the cage and elicits diarrhea.
receptor KO mice indicates that these effects of C75 are unrelated to the function of ghrelin receptors. We found significant correlation between the anorectic and motor activity effects of C75. An overall decrease in motor activity may, in theory, lead to decreased feeding. Although causality cannot be determined with certainty in the present study, it seems unlikely that the anorectic effects are exclusively due to the general CHIR-99021 suppression of motor activity. First, while the overall time-courses correlate significantly, there is a meaningful dissociation between the motor activity- and feeding-suppressive effects of C75. For example, in the ghrelin receptor KO mice, motor activity was unchanged in the first 4 h after C75 Evofosfamide treatment but feeding was already suppressed to,20% of the baseline. Second, if it is the decreased motor activity that interferes with normal feeding behavior after C75 injection then one would expect that the drive for food, i.e., hunger, progressively increases during the dark. Hunger induced by food restriction is accompanied by characteristic changes in sleep-wake activity and c-Fos activation pattern in the brain. Overnight food deprivation results in a,24% increase in waking time during the dark in mice. This arousal response was not observed after C75 injection supporting the notion that the animals were not hungry, in spite of the fact that they ate less than 18% of their normal food amount during the dark period. Also, food deprivation stimulates c-Fos expression in orexigenic brain structures such as the paraventricular nucleus, ARC and LH, but systemic C75 treatment fails to elicit similar activation pattern. A possible explanation for the decreased feeding after C75 injection is that C75 elicits a satiety-like state. The sleep findings, however, do not support this notion. Both naturally occurring satiety that follows feeding as well as injection of satietyinducing hormones such as cholecystokinin lead to increases in sleep. In our study, however, C75 induced dosedependent and long-lasting suppression of REMS. Thus the sleep phenotype after C75 treatment does not match fasting or satiated conditions but shows close similarity to the sleep pattern described in visceral pain models. Visceral illness elicited by LiCl injections is accompanied by transient increase in wakefulness followed by long-lasting suppression of REMS. An ip bolus injection of LiCl causes significant increase in the latency and a significant reduction in the occurrence of REM sleep in the immediate hours following the injection. In contrast, NREM sleep occurrence is only slightly affected by lithium administration. LiCl treatment significantly reduces the relative delta power of the EEG after LiCl treatment. We also observed the suppression of EEG SWA, i.e. delta waves, after C75 administration. Furthermore, LiCl treatment leads to behavioral inactivity and causes rats to lie quietly on the floor of the cage and elicits diarrhea.
Compounds with potentially reactive functionalities inhibitor DAPT strongly inhibited the activation of HSCs
After elimination of weak inhibitors, other undesirable chemical features, as well as compounds for which there were no convenient commercial sources, further validation of the remaining three compounds, candesartan cilexetil, manoalide, and MK-886 were conducted by radioactive gel-based primer extension assays. The results revealed that these compounds were capable of inhibiting the ability of pol k to catalyze synthesis on either a control nondamaged DNA template or a template adducted with the acroleinderived ring-opened reduced form of c-HOPdG in a dosedependent manner with similar potency. Since the predominant role of pol k is in DNA lesion bypass, these results demonstrated that the primer extension assays using damage-containing DNAs can effectively measure the ability of the compounds to inhibit a biologically relevant activity of pol k. In order to assess the ability of these compounds to target intracellular pol k, cell survival assays were carried out by exposing cells to the combination of pol k Tofacitinib inhibitors and UV. The results showed that candesartan cilexetil could potentiate cellular toxicity induced by UV in XP-V cells. It cannot be ruled out that the cellular effect of candesartan cilexetil may be partly due to its effect on other proteins in addition to pol k, including pol g and pol i, since the compound also inhibited the activities of these polymerases in vitro; however, our in vitro results clearly show that pol k is inhibited by this compound. Additionally, it has been shown that the depletion of either pol g or pol i in XP-V cells did not enhance UV cytotoxicity. Collectively, these observations suggest that pol k is inhibited by this compound in the cells, and thus validate the usefulness of this cell-based assay in identifying compounds with potential to inhibit intracellular pol k. Although manoalide and MK-886 could inhibit pol k activity in vitro, these compounds were unable to enhance UV-induced toxicity in XP-V cells under the conditions tested. Both manoalide and MK-886 have anti-inflammatory activity; manoalide is wellknown as a non-specific phospholipase A2 antagonist, and MK-886 inhibits leukotriene synthesis by blocking 5-lipoxygenaseactivating protein. The reason for the inability of these compounds to potentiate UV cytotoxicity could be due to their significantly lower binding affinity to intracellular pol k relative to other cellular targets. Alternatively, these compounds may take a long time to enter the cells and bind to pol k. Moreover, it is possible that only a small fraction of intracellular pol k is inhibited by these compounds and the remaining pol k may be sufficient to process UV-induced DNA lesions, resulting in unaltered cellular sensitivity to UV. Given the presence of multiple back-up TLS polymerases, nearly-complete inhibition of the activity of all intracellular pol k may be essential for cells to present an apparent phenotype. Further understanding of the inability of these compounds to target intracellular pol k could involve 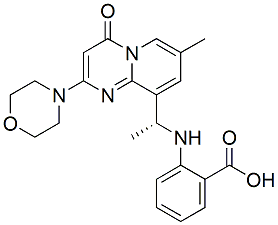 structureactivity relationship analyses. In fact, several structural analogues of these compounds exist such as secomanoalide and luffariellolide for manoalide and L538,916 for MK-886, thus enabling the initiation of such studies. In summary, we presented herein the development of new strategies for the discovery of small molecules that could inhibit pol k activity both in vitro and in vivo. The identification of chemotypes with established drug properties targeting pol k validates this qHTS platform, as well as the secondary assays and sets the stage for exploration of significantly Niraparib larger diverse collections to discover compounds with high potency and specificity towards pol k and thus could potentially be used as pharmaceuticals.
structureactivity relationship analyses. In fact, several structural analogues of these compounds exist such as secomanoalide and luffariellolide for manoalide and L538,916 for MK-886, thus enabling the initiation of such studies. In summary, we presented herein the development of new strategies for the discovery of small molecules that could inhibit pol k activity both in vitro and in vivo. The identification of chemotypes with established drug properties targeting pol k validates this qHTS platform, as well as the secondary assays and sets the stage for exploration of significantly Niraparib larger diverse collections to discover compounds with high potency and specificity towards pol k and thus could potentially be used as pharmaceuticals.
High structural similarity among all HPPK structures suggests developed for advantageous cross-reactivity over many different species
Herein, we report the first structural studies of HPPK from S. aureus using a combination of solution NMR and x-ray crystallographic structure determination, and the identification of a novel pterin-site inhibitor 8-mercaptoguanine byin silico ROCS screening and differential scanning fluorimetry assay. The atomic structure of SaHPPK has been determined in complex with a new pterinsite inhibitor, revealing the molecular details of inhibitor association. Binding of the inhibitor, substrate and cofactor molecules were quantified using isothermal titration calorimetry and surface plasmon resonance, while in vitro enzyme inhibition data was WZ4002 1213269-23-8 measured using a luciferase based luminescent assay. Detailed studies of ligand interactions using NMR highlight critical ligand-induced dynamic changes upon inhibitor, substrate and cofactor binding, which correlate with large entropic penalties to the binding thermodynamics of the inhibitor measured by ITC. Titration of 8-mercaptoguanine into a sample of the apo enzyme produced a range of CSPs and exchange regimes in the NMR spectra. 15 cross peaks broadened completely around the binding site and along the sheet and most others exhibited 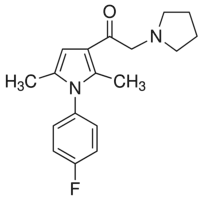 slow exchange, indicative of a Kd likely in the low mM range. In contrast, when performed in the presence of saturating ATP or AMPCPP, widespread perturbations were observed in slow exchange for all resonances, despite no change in binding affinity measured by ITC and SPR. Chemical shift perturbations clearly mapped to the respective substrate and cofactor site. This work reports the discovery, binding properties and mechanism of a novel, competitive pterin site inhibitor, presented in complex with the first crystal structure of SaHPPK. The pterin site is highly specific and restricts the chemical space available for inhibitor design to structures closely resembling the pterin scaffold. Consequently, the literature is devoid of non-pterin like HPPK inhibitors, despite mounting structural information that has been reported over the last decade. In line with the high pterin-site specificity is the high ligand efficiency of 8-mercaptoguanine. 8-Mercaptoguanine has previously been reported to have biological activity. Early studies revealed some lipolytic PF-4217903 activity while in a number of cases 8-mercaptoguanine has been shown to inhibit enzymes that normally bind purines. Antiviral activity,without significant toxicity, was also reported in an in vivo mouse model. Close analogues, such as 8-mercaptoguanosine, were also shown to induce interleukin-1 activity in macrophages. Despite these studies, no antibacterial activity has been reported previously. Interestingly, 8mercaptoguanine has been shown to bind to, but not inhibit, B. anthracis DHPS by co-crystallisation, which may open the possibility for a multi target inhibitor derived from this scaffold. In the present work, we did not observe growth inhibition in vivo by 8mercaptoguanine in E. coli cell-based assays. Given the unfavourable logP, this is likely to be due to poor membrane permeability. This may be a disadvantage for pterin-like inhibitors in general given the hydrophilic nature and restrictive chemical space of the pterin scaffold in folate pathway enzymes. Nevertheless, while insufficient transport of a set of closely related pyrimidines as potential antifolates was implicated in their poor in vivo inhibition, derivatives with an additional phenyl substituent displayed sub micromolar activity in vivo to T. brucei and L. major. The known phenethyl in vitro inhibitor of HPPK suggests that a suitably positioned phenyl group on 8-mercaptoguanine may thus be beneficial to both binding and assist cell permeability.
slow exchange, indicative of a Kd likely in the low mM range. In contrast, when performed in the presence of saturating ATP or AMPCPP, widespread perturbations were observed in slow exchange for all resonances, despite no change in binding affinity measured by ITC and SPR. Chemical shift perturbations clearly mapped to the respective substrate and cofactor site. This work reports the discovery, binding properties and mechanism of a novel, competitive pterin site inhibitor, presented in complex with the first crystal structure of SaHPPK. The pterin site is highly specific and restricts the chemical space available for inhibitor design to structures closely resembling the pterin scaffold. Consequently, the literature is devoid of non-pterin like HPPK inhibitors, despite mounting structural information that has been reported over the last decade. In line with the high pterin-site specificity is the high ligand efficiency of 8-mercaptoguanine. 8-Mercaptoguanine has previously been reported to have biological activity. Early studies revealed some lipolytic PF-4217903 activity while in a number of cases 8-mercaptoguanine has been shown to inhibit enzymes that normally bind purines. Antiviral activity,without significant toxicity, was also reported in an in vivo mouse model. Close analogues, such as 8-mercaptoguanosine, were also shown to induce interleukin-1 activity in macrophages. Despite these studies, no antibacterial activity has been reported previously. Interestingly, 8mercaptoguanine has been shown to bind to, but not inhibit, B. anthracis DHPS by co-crystallisation, which may open the possibility for a multi target inhibitor derived from this scaffold. In the present work, we did not observe growth inhibition in vivo by 8mercaptoguanine in E. coli cell-based assays. Given the unfavourable logP, this is likely to be due to poor membrane permeability. This may be a disadvantage for pterin-like inhibitors in general given the hydrophilic nature and restrictive chemical space of the pterin scaffold in folate pathway enzymes. Nevertheless, while insufficient transport of a set of closely related pyrimidines as potential antifolates was implicated in their poor in vivo inhibition, derivatives with an additional phenyl substituent displayed sub micromolar activity in vivo to T. brucei and L. major. The known phenethyl in vitro inhibitor of HPPK suggests that a suitably positioned phenyl group on 8-mercaptoguanine may thus be beneficial to both binding and assist cell permeability.
Collectively our observations imply that the previously reported that L-PDMP which activates LacCer synthase
In endothelial cells can also induce angiogenensis in a dose-dependent manner and also RENCA cell proliferation in the present study. Thus activation/ inactivation of LacCer synthase by agonists/antagonists may well regulate angiogenesis in vitro and in vivo. Our BIBW2992 studies and those of others have shown that D-PDMP is non-toxic when given at doses ten times that of the concentration used in the present study. The body weight in this study did not differ in placebo vs. D-PDMP�Ctreated mice. The tumor weight decreased approximately 50% in 3 MPK and 10 MPK fed mice compared to placebo. However, when mice were fed higher amounts of D-PDMP; 25 and 50 MPK, it did not further reduce tumor volume. In a previous study, it was shown that the t1/2 of D-PDMP in mice blood is,50 min. Consequently, it is feasible that 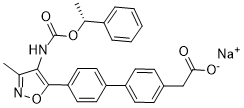 beyond a threshold of 10 MPK, most of this compound is rapidly removed by excretion and therefore further reduction in tumor volume was not observed. Previously, D-PDMP has been used extensively to examine the role of glycosphingolipid and related glycosytransferases in arterial smooth muscle cell proliferation, wound healing, osteoclastogenesis, Nutlin-3 polycystic kidney disease, elasticity, respiratory diseases, glioblastoma research cholesterol efflux, inflammation in vitro and in vivo, shear stress, and A beta secretion in neuroblasotma cells. Although D-PDMP is known to inhibit the activity of UGCG, raise ceramide levels and induce cell death by apoptosis-we could not reproduce these observations in vivo in mice kidney. In agreement with a previous study we also observed that the level of ceramide in kidney in D-PDMP �Ctreated mice was lower. Likewise, an iminosugar, another inhibitor of UGCG also did not raise the level of ceramide in a transgenic mouse model of hyperlipidemia. Moreover, in a recent study, the use of another glucosylceramide synthase inhibitor, Genz122346, in a mouse model of polycystic kidney disease revealed that this compound also inhibits proliferation but does not inhibit apoptosis involving ceramide. This could be due to further catabolism of ceramide as the activity of several hydrolases including ceramide deacylase maybe higher upon treatment with D-PDMP. Also ceramide may be converted to other sphingolipids. These observations attest to the multiple fates of ceramide and multiple pools of ceramide in kidney tissue. Indeed, we observed that the activity of GlcCer glucosidase was increased in D-PDMP�Ctreated mice compared to placebo. This may have contributed to an increase in the level of GlcCer in mice fed D-PDMP. We have previously shown that in cultured human arterial endothelial cells, D-PDMP can inhibit VEGF-induced angiogenesis and this was bypassed by LacCer but not S-1-P. Such observations suggest that LacCer mediated and VEGF-induced angiogenesis is independent of S-1-P-induced angiogenesis. Moreover, use of 1-phenyl-2-palmitoylamino-3-morpholino-1propanol ; a relatively more specific inhibitor of GlcCer synthase compared to D-PDMP to mitigate VEGF induced angiogenesis was bypassed by feeding LacCer in human endothelial cells. In fact VEGF-induced angiogenensis in these cells were mitigated,1.5 fold better by the use of D-PDMP compared to PPMP. Finally, LCS gene ablation by the use of siRNA mitigated VEGF induced angiogenesis in these cells. In the present study, we document that D-PDMP may well inhibit angiogenesis by way of mitigating the expression of p-AKT-1 and mTOR expression in mice kidney.
beyond a threshold of 10 MPK, most of this compound is rapidly removed by excretion and therefore further reduction in tumor volume was not observed. Previously, D-PDMP has been used extensively to examine the role of glycosphingolipid and related glycosytransferases in arterial smooth muscle cell proliferation, wound healing, osteoclastogenesis, Nutlin-3 polycystic kidney disease, elasticity, respiratory diseases, glioblastoma research cholesterol efflux, inflammation in vitro and in vivo, shear stress, and A beta secretion in neuroblasotma cells. Although D-PDMP is known to inhibit the activity of UGCG, raise ceramide levels and induce cell death by apoptosis-we could not reproduce these observations in vivo in mice kidney. In agreement with a previous study we also observed that the level of ceramide in kidney in D-PDMP �Ctreated mice was lower. Likewise, an iminosugar, another inhibitor of UGCG also did not raise the level of ceramide in a transgenic mouse model of hyperlipidemia. Moreover, in a recent study, the use of another glucosylceramide synthase inhibitor, Genz122346, in a mouse model of polycystic kidney disease revealed that this compound also inhibits proliferation but does not inhibit apoptosis involving ceramide. This could be due to further catabolism of ceramide as the activity of several hydrolases including ceramide deacylase maybe higher upon treatment with D-PDMP. Also ceramide may be converted to other sphingolipids. These observations attest to the multiple fates of ceramide and multiple pools of ceramide in kidney tissue. Indeed, we observed that the activity of GlcCer glucosidase was increased in D-PDMP�Ctreated mice compared to placebo. This may have contributed to an increase in the level of GlcCer in mice fed D-PDMP. We have previously shown that in cultured human arterial endothelial cells, D-PDMP can inhibit VEGF-induced angiogenesis and this was bypassed by LacCer but not S-1-P. Such observations suggest that LacCer mediated and VEGF-induced angiogenesis is independent of S-1-P-induced angiogenesis. Moreover, use of 1-phenyl-2-palmitoylamino-3-morpholino-1propanol ; a relatively more specific inhibitor of GlcCer synthase compared to D-PDMP to mitigate VEGF induced angiogenesis was bypassed by feeding LacCer in human endothelial cells. In fact VEGF-induced angiogenensis in these cells were mitigated,1.5 fold better by the use of D-PDMP compared to PPMP. Finally, LCS gene ablation by the use of siRNA mitigated VEGF induced angiogenesis in these cells. In the present study, we document that D-PDMP may well inhibit angiogenesis by way of mitigating the expression of p-AKT-1 and mTOR expression in mice kidney.